How to Apply
-
Intent
-
Develop a sample
-
Analysis plan to gather data in support of your process's equivalency
-
Carry out that sampling plan and
-
Complete and submit an official pathogen reduction equivalency application package.
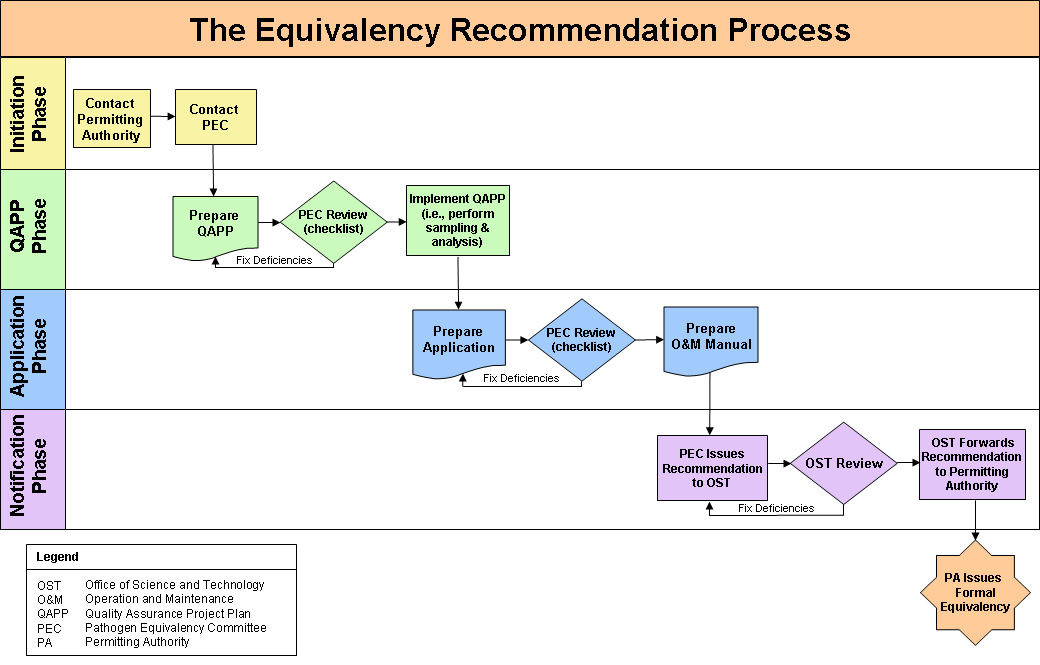
The Pathogen Equivalency Committee will then evaluate the package and make its recommendation through the appropriate channels. This typical equivalency recommendation process is shown in this flow diagram.
Resources
Listed are some resources to help applicants submit an application package to obtain a recommendation for equivalency to a Process to Further Reduce Pathogens (PFRPs) or a Process to Significantly Reduce Pathogens (PSRP).
A QAPP ensures that the desired quality in sample collection, laboratory analysis, data validation & reporting, and documentation & record-keeping are achieved and maintained.
Please use this link to identify your permitting authority and access their contact information.
This checklist is used by the PEC to review submitted QAPPs and equivalency applications. It is provided to help applicants double-check that all required and applicable elements have been addressed in their QAPP/equivalency application before submittal.
A detailed outline for a full application for an equivalency recommendation. The outline is annotated, providing specific information appropriate for each section.
