Herbicides
Overview
Herbicides are chemicals used to manipulate or control undesirable vegetation. Herbicide application occurs most frequently in row-crop farming, where they are applied before or during planting to maximize crop productivity by minimizing other vegetation. They also may be applied to crops in the fall, to improve harvesting.
Reproduced from USDA Economic Research Service, Pest Management
Herbicides are used in forest management to prepare logged areas for replanting. The total applied volume and area covered is greater but the frequency of application is much less than for farming (Shepard et al. 2004).
In suburban and urban areas, herbicides are applied to lawns, parks, golf courses and other areas. Herbicides are applied to water bodies to control aquatic weeds. These weeds can impede irrigation withdrawals or interfere with recreational and industrial uses of water (Folmar et al. 1979).
The potential effects of herbicides are strongly influenced by their toxic mode of action and their method of application. The molecular site of action is challenging to predict because structural associations have not been identified (Duke 1990), but modes of action are well-established.
Herbicides can act by inhibiting cell division, photosynthesis or amino acid production or by mimicking natural plant growth hormones, causing deformities (Ross and Childs 1996). Application methods include spraying onto foliage, applying to soils and applying directly to aquatic systems.
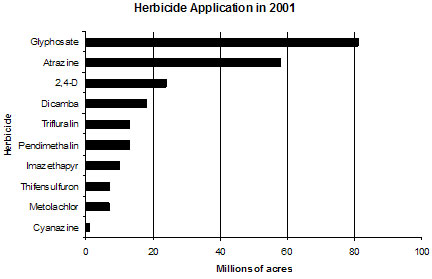
Figure 1 and Table 1 present the ten herbicides most used on agricultural land in the U.S. Glyphosate and atrazine were applied to more than double the crop field acreage than the third leading herbicide, 2,4-D, in 2001.
| Herbicide | Common Application | Mode of Action |
|---|---|---|
| Amino Acid Inhibitors | ||
| Glyphosate Roundup, Ultra, Rodeo, TouchDown Pro, Accord |
Applied primarily to genetically engineered, glyphosate-resistant varieties of soybeans, corn, canola and cotton. Also applied to control woody plants. Because of its broad spectrum and relatively low toxicity to animals, it is used in horticulture and in the control of aquatic macrophytes. | Applied to the foliage and transported with sugars to metabolic sites where they inhibit amino acid production. Effects will manifest in two or more weeks as discoloration of foliage and deformations in new growth. |
| Imazethapyr Pursuit |
Applied to control weeds in alfalfa, barley, soybeans and wheat. | |
| Thifensulfuron Beacon, Pinnacle, Harmony |
Applied to control weeds in small grains, soybeans and corn and in conifer and hardwood plantations. | |
| Photosynthesis Inhibitors | ||
| Atrazine Aatrex, Atrazine |
Applied to crops such as corn, soybeans and sorghum, particularly for conservation tillage. | These broad spectrum herbicides are applied at the soil and carried to the leaves by transpiration. They inhibit photosynthesis. |
| Cyanazine Bladex/DuPont |
||
| Synthetic Auxin, Growth Regulators | ||
| 2,4-D | Applied to broad-leafed weeds in corn, small grains, sorghum, pastures and rangeland. Urban use on lawns and grassy rights of way. Also applied for control of broad-leafed trees when planting conifers. | These synthetic growth hormones are applied to the foliage of dicots and transported to meristems causing uncontrolled growth. Effects can be observed as discoloration of foliage and deformations in new growth. They are fast acting: effects on foliage are visible within minutes of application. |
| Dicamba Banvel, Clarity, Vanquish, Veteran |
||
| Cell Division Inhibitors | ||
| Trifluralin Treflan and others |
Applied to control grasses and broadleaf weeds in crops such as beans, peanuts, cotton and tobacco. | These herbicides are applied to the soil to control target vegetation before emergence by inhibiting root growth. |
| Pendimethalin Prowl, Pentagon, Pendulum, Stomp |
||
| Metolachlor Dual, Dual Magnum, Pennant Magnum |
Applied pre-planting to control annual grasses and broadleaf weeds among crops such as corn and soybeans. | This herbicide is applied to the soil to control target vegetation by inhibiting or disrupting cell division in shoots. |
| Adapted from Ross and Childs (1996) and USDA; commercial names in italics. | ||
Herbicides may cause biological impairments of water bodies if they occur in water or sediment at sufficient concentrations. Most commonly, they enter surface water in runoff or leachate, but, because they have relatively low toxicity to fish and invertebrates (see Table 2). Acute toxicity is likely only when they are deliberately or accidentally applied directly to water bodies.
| Herbicide | Taxa | Biological Effect |
|---|---|---|
| Glyphosate | ||
| Water flea Daphnia magna |
Acute 48h EC50 is 218 mg/L (ECOTOX) | |
| Amphipod Gammarus pseudolimnaeus |
Acute 48h EC50 is 42-62 mg/L (ECOTOX) | |
| Buzzer midge Chironomus plumosus |
Acute 48h EC50 is 55 mg/L technical glyphosate and 13mg/L Roundup® surfactant (Folmar et al. 1979) | |
| Mayfly Ephemerella walkeri |
Avoided Roundup® at 10 mg/L but not 1.0 mg/L (Folmar et al. 1979) | |
| Channel catfish Ictalurus punctatus |
Acute 96h LC50 is 130mg/L technical glyphosate and 13mg/L Roundup® surfactant (Folmar et al. 1979) | |
| Fathead minnow Pimephales promelas |
Acute 96h LC50 is 97mg/L technical glyphosate and 1.0 mg/L Roundup® surfactant (Folmar et al. 1979) | |
| Rainbow trout Oncorhynchus mykiss |
More sensitive response to Roundup® at elevated temperatures and at pH as it rises from 6.5 to 7.5, with no increased sensitivity at pH beyond 7.5 (Folmar et al. 1979) | |
| Bluegill sunfish Lepomis macrochiru |
||
| American ribbed fluke snail Pseudosuccinia columella |
Continuous exposure across generations produced reproductive effects on the third generation including rapid embryonic development, embryonic abnormalities and increased egg laying (Tate et al. 1997) | |
| Atrazine | ||
| Midge Labrundinia pilosella |
Reduced emergence at 20 ug/L (Dewey 1986) | |
| Cream and brown microcaddisfly Oxyethira pallida |
Shift in emergence period at 20 ug/L (Dewey 1986) | |
| Non-predatory insects | Reduced abundance at 20 ug/L (Dewey 1986) | |
| Stonewort algae Chara sp. |
Resistant to atrazine up to 100 ug/L (Dewey 1986) | |
| Tiger salamander Ambystoma tigrinum sp. |
Increased larval stage duration, reduced weight and body size (Larson et al. 1998) | |
| Hydra sp. | 48 hr LC50 of 3,000 ug/L (lowest acute value) (U.S. EPA 2003) | |
| Goldfish Carassius auratus |
96 hr LC50 of 60,000 ug/L (highest acute value) (U.S. EPA 2003) | |
| Water flea Ceriodaphnia dubia |
Life cycle chronic value of 3,536 ug/L (highest chronic value) (U.S. EPA 2003) | |
| Brook trout Salvelinus fontinalis |
Life cycle chronic value of 88.32 ug/L (lowest chronic value) (U.S.EPA 2003) | |
Direct applications may result in direct toxicity to non-target plants and animals or indirect effects due to the death and decomposition of plants. Impairments also are more likely when herbicides are applied together or with other pesticides (Streibig et. al. 1998), resulting in additive or synergistic effects.
Atrazine reacts synergistically with chlorpyrifos: the mixture was seven times more toxic to an earthworm species than the two individual pesticides (Lydy and Linck 2003). Atrazine also increased the effects of other pesticides in mosquito larvae and various flies (Belden and Lydy 2000, Lydy and Linck 2003). The surfactants used in herbicide solutions also can be toxic to biota and are not considered when testing active ingredients (Folmar et al. 1979).
Checklist of Sources, Site Evidence and Biological Effects
Herbicides are addressed in this module as proximate stressors. Herbicides should be a candidate cause when human sources and activities, site observations or observed effects support portions of the causal pathways (see Figure 2). The conceptual diagram and other information also may be useful in Step 3: Evaluate Data from the Case.
Rather than causing direct toxicity to organisms, herbicides may contribute to other stressors (e.g., instream habitat alteration via riparian devegetation). In such cases, herbicides can be considered as part of the pathway for the proximate cause of impairment.
The checklist below will help you identify key data and information useful for determining whether to include herbicides among your candidate causes. This list is intended to guide you in collecting evidence to support, weaken or eliminate herbicides as a candidate cause.
For more information on specific entries, go to the When to List tab.
Sources and Activities
- Forest management
- Agriculture/crop cultivation
- Parks
- Golf courses
- Lawns
- Roads/rights of way
- Aquatic weed control
Site Evidence
- Dead or injured plants
- Fish kills
- Irrigation channels
- Drainage from fields or lawns
- Orchards
Biological Effects
- Inhibition of phytoplankton, periphyton or macrophytes
- Reduced invertebrate species richness and abundance
- Reduction of sensitive species and abundance of tolerant species
Other Stressors that May Influence Herbicide Effects
- Temperature: Elevated temperature frequently tends to increase the toxicity of chemicals.
- Moderate to high pH: pH will determine the ionic state and bioavailability of ionizable herbicides.
- Dissolved oxygen (DO): Signs of herbicide application are evidence of a causal pathway to low DO when DO is a candidate cause of a kill or other impairment.
- Unspecified toxics: Surfactants in herbicide formulations can be more toxic to animals than the active ingredients (Folmar et al. 1979, Diamond and Durkin 1997). The toxicity of Roundup®, which increased with elevated pH, was attributed to the surfactant, rather than the active ingredient, glyphosate (Folmar et al. 1979). This result is reinforced by findings that more alkaline pH decreases glyphosate toxicity but increases surfactant toxicity (Diamond and Durkin 1997).
You also may wish to consider other causes with similar evidence:
- Insecticides
- Endocrine disruptors
- Toxic metals
When to List
- Sources and Activities that Suggest Listing Herbicides as a Candidate Cause
- Site Evidence that Suggests Listing Herbicides as a Candidate Cause
- Biological Effects that Suggest Listing Herbicides as a Candidate Cause
- Site Evidence that Supports Excluding Herbicides as a Candidate Cause
Sources and Activities that Suggest Listing Herbicides as a Candidate Cause
Forestry management practices, agricultural operations, and urban development and maintenance are all sources of herbicides that may enter surface waters and cause impairments. Herbicides are applied to forests after harvesting to suppress brush and noncommercial trees. For that use, the rate of application may be high and exposed streams are more likely to be of higher quality than agricultural or urban streams. Conversely, agricultural operations may contribute large quantities of herbicides because they may apply herbicides multiple times per year and they may be applied by planes, addition to irrigation water or spraying onto crops (see Figure 3).

Urban land uses can contribute as homeowners and managers of parks, golf courses and other lawns use herbicides for aesthetic enhancement. Herbicides also are used on rights of way for roads, pipelines, railroads and electrical transmission lines and for control of plants in cracks in pavements. Such urban and suburban uses are likely to contaminate storm waters.
Herbicides also are directly applied to waters to control vegetation in ponds, ditches, irrigation canals and recreational waters. Such applications are sources of exposure at the point of application and downstream.
Site Evidence that Suggests Listing Herbicides as a Candidate Cause

Although herbicides in general have lower toxicity to animals than other pesticides, fish or invertebrate kills may be a sign of herbicide use. For example, acrolein has been applied to irrigation ditches at levels sufficient to be acutely lethal to fish and invertebrates (see acrolein in U.S. EPA 2009), and if not properly applied to fields it can cause kills in receiving waters. Kills also may be due to low dissolved oxygen (DO) concentrations resulting from plant materials decomposing in water.
Biological Effects that Suggest Listing Herbicides as a Candidate Cause
Because herbicides tend to affect plants more quickly and severely than animals, the most useful biological sign of herbicides is effects on aquatic plants (Kreutzweiser et al. 1995, Van den Brink et al. 1997, Hall et al. 1997). This trait may help distinguish the biological effects of herbicides from those of insecticides and most other toxic chemicals. Secondary effects of herbicides are mediated by low DO concentrations from plant decomposition and changes in trophic structure due to plant community changes.
Herbicides may reduce taxa richness and abundance of fish and benthic macroinvertebrates due to reductions of sensitive species and increased abundance of tolerant species at high concentrations (Daam and Van den Brink 2007, Dewey 1986). It also has been contended that some herbicides, particularly atrazine, have specific mechanisms of action in aquatic frogs and fish, including developmental abnormalities (Hayes et al. 2006, Tillit et al. 2010). However, a review by the U.S. EPA found that evidence for such effects in amphibians was weak and inconsistent (U.S. EPA 2007).
Site Evidence that Supports Excluding Herbicides as a Candidate Cause

Ways to Measure
Herbicides and their metabolites can be measured in groundwater and surface water by gas chromatography (GC), mass spectrometry (MS), high performance liquid chromatography with diode-array detection (HPLC/DAD), liquid chromatography (LC), solid-phase extraction (SPE) or enzyme-linked immunosorbent assay (ELISA) (Scribner et al. 2000). Because there isn't a standard method for detecting all herbicides, measurements can be difficult, expensive and time-consuming.
Herbicide metabolites can have toxicity similar to that of the parent herbicide and are often found in higher concentrations (USGS 2010). Presently metabolites of triazines, chloroacetanilides, phenyl ureas and the phosphanoglycine glyphosate have been measured (Scribner et al. 2000, USGS 2010). Different herbicides and metabolites are measurable using different techniques, and the proper technique must be matched with the metabolite of interest. The USGS Toxic Substances Hydrology Program provides guidance, lab methods, field methods and literature related to detecting herbicides in ground and surface water.
Conceptual Diagrams
About Conceptual Diagrams
Conceptual diagrams are used to describe hypothesized relationships among sources, stressors and biotic responses within aquatic systems.
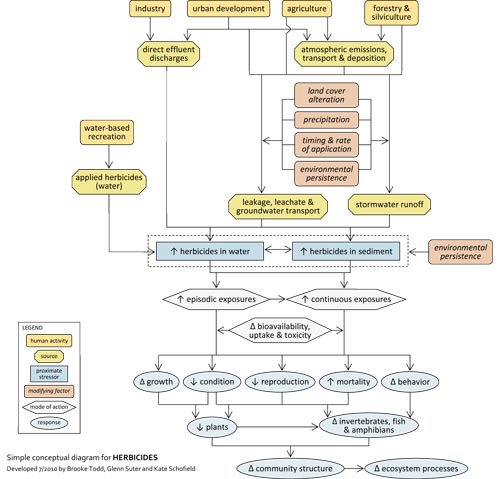
Simple Conceptual Model Diagram
Herbicides are used to control undesired plants on farms, in commercial forests, and on lawns and managed landscapes. Herbicides are sometimes applied directly to surface water for aquatic weed control. Typically herbicides are applied to soil or terrestrial vegetation, which can increase herbicides in groundwater discharge, atmospheric drift and runoff. The extent to which herbicides reach streams depends on factors such as precipitation, application timing and rates and environmental persistence of herbicides and their metabolites.
In streams, herbicides may be dissolved in the water column or bound to sediments, and their impact depends on the medium in which they occur. Exposures may be episodic (e.g., occurring during runoff events) or continuous (e.g., exposure to herbicide contaminated bed sediments). The bioavailability, uptake and toxicity of herbicides vary with environmental conditions (e.g., pH).
Increased herbicides in streams can adversely affect stream flora and fauna via several mechanisms, including reduced growth, condition, and reproduction; increased mortality; and changes in behavior. These effects can result in biologically impaired macrophyte, periphyton, phytoplankton, fish and invertebrate assemblages, which in turn can contribute to changes in community structure and ecosystem function.
Detailed Conceptual Model Diagram
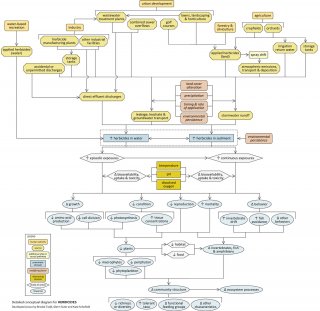
High concentrations of herbicides and their metabolites in streams can have lethal and sub-lethal effects on aquatic biota, potentially changing community structure and ecosystem function. This conceptual diagram (Figure 7) illustrates linkages between human activities and sources (top of diagram), herbicide-related stressors (middle of diagram), and the biological responses that can result (bottom of diagram).
In some cases, additional steps leading from sources to stressors, modes of action leading from stressors to responses, and other modifying factors are shown. This narrative generally follows the diagram top to bottom, left to right.
Linking Sources to Stressors
Anthropogenic activities and land uses, such as industry, urban development, forestry and agriculture can contribute herbicides to streams. Herbicide manufacturers, industrial facilities and wastewater treatment plants may discharge effluents containing herbicides. Accidental or unpermitted discharges also may occur.
Herbicides are sometimes applied directly to surface water for aquatic weed control (e.g., for water-based recreation). Herbicides may be applied to golf courses, lawns and other managed landscapes, forests, crop fields and orchards to control a variety of unwanted vegetation. In some cases, herbicides may be transported atmospherically in spray drift. These applied herbicides may enter streams via stormwater runoff, groundwater discharges or direct atmospheric deposition.
Stored herbicides, both at sites where they are used and at sites where they are manufactured, also may be transported to streams via runoff or groundwater transport. The extent to which these transport pathways occur depends upon several factors, including land cover, precipitation patterns, timing and rates of application and environmental persistence of the herbicides.
Linking Stressors to Biological Responses
In streams, herbicides can be dissolved in the water column or bound to sediments, and the effects they have will depend upon the medium in which they occur. Exposures may be episodic (e.g., pulsed deliveries with stormwater runoff) or continuous (e.g., long-term exposure to herbicide-contaminated sediments). The bioavailability, uptake, and toxicity of herbicides and their metabolites during these exposures depends on factors such as temperature, pH, and dissolved oxygen concentrations.
The most direct effects of herbicide pollution are decreased condition, growth, and reproduction, and increased mortality, of plants (i.e., macrophytes, periphyton and phytoplankton). For example, exposure to herbicides may lead to elevated internal herbicide concentrations and decreased photosynthesis, cell division, and amino acid production in plants. Effects on aquatic plants can indirectly affect fish and invertebrates by modifying habitat and food availability.
Exposure to herbicides also can directly increase mortality and change the behavior and reproduction of fish, amphibians and invertebrates. Possible changes in behavior include increased invertebrate drift and increased avoidance by fish.
Ultimately, these effects may result in changes in community structure (e.g., decreased richness, changes in functional feeding groups) and ecosystem function. For example, aquatic vegetation is especially susceptible to herbicides, so may decrease in abundance and richness. As a result, the relative abundances of invertebrate feeding groups may shift. However, herbicide-resistant and other non-target plants may increase in abundance with herbicide exposure, due to reduced competitive pressure from affected plants.
Literature Reviews
This section presents an annotated bibliography of references providing information on stressor-response relationships for herbicides, as well as general background on herbicide properties. This is not meant to be a comprehensive bibliography of references dealing with herbicides, but rather is meant to highlight a few references that may be especially useful.
- Kegley SE, Hill BR, Orme S, Choi AH (2010) Pesticide Action Network Pesticide Database. Pesticide Action Network, North America, San Francisco CA.
This database has toxicity data for pesticides across many species. It provides a good starting point for finding pesticide use, occurrence, and effects data on the web.
- Ross MA, Childs DJ (1996) Herbicide Mode-of-Action Summary. Purdue University, Department of Botany: Plant Pathology, West Lafayette IN. Report No. WS-23-W.
This publication provides a breakdown of seventy-eight common herbicides organized by translocation mechanism and then mode of action. It further subdivides the information into chemical type and then common and trade names. A brief paragraph describes each mode of action and types of vegetation that the herbicide is often used to control.
- Stenersen J (2009) Chemical Pesticides: Mode of Action and Toxicology. CRC Press, Boca Raton FL.
This is a recent reference for mechanistic health and environmental toxicity information for pesticides, including herbicides and insecticides.
- U.S. EPA (2009) Aquatic Life Benchmarks for Pesticide Registration. U.S. Environmental Protection Agency, Office of Pesticide Programs, Washington DC.
The aquatic life benchmarks (for freshwater species) provided in this module are based on toxicity values reviewed by U.S. EPA and used in the Agency's most recent risk assessments, developed as part of the decision-making process for pesticide (including herbicides) registration. Acute and chronic benchmarks are provided for fish, invertebrates and aquatic plants. The table of benchmarks provides links to supporting ecological risk assessments. Each aquatic life benchmark is based on the most sensitive, scientifically acceptable toxicity endpoint available to U.S. EPA for a given taxon. U.S. EPA's goal is to add to these benchmarks annually.
References
- Belden J, Lydy MJ (2000) Impact of atrazine on organophosphate insecticide toxicity. Environmental Toxicology and Chemistry 19:2266-2274.
- Daam MA, Van den Brink PJ (2007) Effects of Chlopyrifos, Carbendazim, and Linuron on the ecology of a small indoor aquatic microcosm. Archives of Environmental Contamination and Toxicology 53(1):22-35.
- Dewey SL (1986) Effects of the herbicide atrazine on aquatic insect community structure and emergence. Ecology 67(1):148-162.
- Diamond GL, Durkin PR (1997) Effects of Surfactants on the Toxicity of Glyphosate, with Specific Reference to RODEO. U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Riverdale MD. SERA TR 97-206-1b.
- Duke SO (1990) Overview of herbicide mechanisms of action. Environmental Health Perspectives 87:263-271.
- Folmar LC, Sanders HO, Julin AM (1979) Toxicity of the herbicide glyphosate and several of its formulations to fish and aquatic invertebrates. Archives of Environmental Contamination and Toxicology 8:269-278.
- Hall LW Jr, Anderson RD, Ailstock MS (1997) Chronic toxicity of atrazine to sago pondweed at a range of salinities: implications for criteria development and ecological risk. Archives of Environmental Contamination and Toxicology 33:261-267.
- Hayes TB, Stuart AA, Mendoza M, Collins A, Noriega N, Vonk A, Johnston G, Liu R, Kpodzo D (2006) Characterization of atrazine-induced gonadal malformations in African clawed frogs (Xenopus laevis) and comparisons with effects of an androgen antagonist (cyproterone acetate) and exogenous estrogen (17B-estradiol): support for the demasculinization/feminization hypothesis. Environmental Health Perspectives 114(Supplement 1):134-141.
- Kegley SE, Hill BR, Orme S, Choi AH (2010) Pesticide Action Network Pesticide Database. Pesticide Action Network, North America, San Francisco CA.
- Kreutzweiser DP, Capell SS, Sousa BC (1995) Hexazinone effects on stream periphyton and invertebrate communities. Environmental Toxicology and Chemistry 14(9):1521-1527.
- Larson DL, McDonald S, Fivizzani AJ, Newton WE, Hamilton SJ (1998) Effects of the herbicide atrazine on Ambystoma tigrinum metamorphosis: duration, larval growth, and hormonal response. Physiological Zoology 71(6):671-679.
- Lydy MJ, Linck SL (2003) Assessing the impact of triazine herbicides on organophosphate insecticide toxicity to the earthworm Eisenia fetida. Archives of Environmental Contamination and Toxicology 45:343-349.
- Ross MA, Childs DJ (1996) Herbicide Mode-of-Action Summary. Purdue University, Department of Botany: Plant Pathology, West Lafayette IN. Report No. WS-23-W.
- Scribner EA, Thurman EM, Zimmerman LR (2000) Analysis of selected herbicide metabolites in surface and ground water of the United States. Science of the Total Environment 248(2-3):157-167.
- Shepard JP, Creighton J, Duzan H (2004) Forestry herbicides in the United States: an overview. Wildlife Society Bulletin 32(4):1020-1027.
- Stenersen J (2009) Chemical Pesticides: Mode of Action and Toxicology. CRC Press, Boca Raton FL.
- Streibig JC, Kudsk P, Jensen JE (1998) A general joint action model for herbicide mixtures. Pesticide Science 53(1):21-28.
- Tate TM, Spurlock JO, Christian FA (1997) Effect of glyphosate on the development of Pseudosuccinea columella snails. Archives of Environmental Contamination and Toxicology 33:286-297.
- Tillit DE, Papoulias DM, Whyte JJ, Richter CA (2010) Atrazine reduces reproduction in fathead minnow (Pimephales promelas). Aquatic Toxicology 99(2):149-159.
- U.S. EPA (2003) Ambient Aquatic Life Water Quality Criteria for Atrazine: Revised Draft. Office of Water, Office of Science and Technology, Health and Ecological Criteria Division, Washington DC. EPA-822-R-03-023.
- U.S. EPA (2007) White Paper on the Potential for Atrazine to Affect Amphibian Gonadal Development. U.S. Environmental Protection Agency, Office of Pesticide Programs, Washington DC.
- U.S. EPA (2009) Aquatic Life Benchmarks for Pesticide Registration. U.S. Environmental Protection Agency, Office of Pesticide Programs, Washington DC.
- U.S. EPA (2009) Ambient Aquatic Life Water Quality Criteria for Acrolein. Office of Water, Office of Science and Technology, Health and Ecological Criteria Division, Washington DC. CAS Registry No. 107-02-8. EPA/822/R-09/010
- USGS (2010) Glyphosate herbicide found in many midwestern streams, antibiotics not common. U.S. Geological Survey.
- Van den Brink PJ, Crum SJH, Glystra R, Bransen F, Cuppen JGM, Brock TCM (2009) Effects of a herbicide-insecticide mixture in freshwater microcosms: risk assessment and ecological effect chain. Environmental Pollution 157:237-249.
- Van den Brink PJ, Hartgers EM, Fettweis U, Crum SJH, Van Donk E, Brock TCM (1997) Sensitivity of macrophyte-dominated freshwater microcosms to chronic levels of the herbicide Linuron. Ecotoxicology and Environmental Safety 38:13-24.
Contacts: Authors & Contributors