Long-Lasting Disinfection Evaluation Test Results August 4, 2020
August 4, 2020
Experimental Approach
A standard method does not currently exist for testing residual efficacy against viruses on surfaces. A standardized approach was developed to achieve the objective of this project by combining elements of ASTM 2197-17, ATSM 1053, and EPA’s “Protocol for the Evaluation of Bactericidal Activity of Hard, Non-porous Copper Containing Surface Product”.
In general, uniform pieces of surface material (coupons) were prepared, cleaned, rinsed, sterilized, and coated with the product being tested in accordance with product manufacturer’s directions, and allowed to dry overnight. On the following day, testing “Day 0”, virus was spiked onto product-coated (coated) and uncoated coupons. Sets of coupons (three coated and three uncoated) were then extracted to recover infective virus at defined time points (2, 4, 8, 24, and 48 hours contact time) after inoculation. Infectious virus was quantitatively determined using methods appropriate for each virus (see Table 1) and described in the approved Quality Assurance Project Plans (QAPPs). The impact of the material and the product on analysis methods was assessed for each method and described in the QAPPs.
Table 1: Virus Types used in this Study
|
Virus |
Virus Description |
Viral Host |
Analysis Method |
|---|---|---|---|
|
MS2 (ATCC 15597-B1) |
Non-enveloped virus; bacteriophage |
Escherichia coli |
Plaque assay, E. coli C-300 (ATCC 15597) |
|
Phi6 |
Enveloped virus; bacteriophage |
Pseudomonas syringae |
Plaque assay, P. syringae LM2489 |
|
MHV-A59 |
Enveloped virus; beta coronavirus |
Mouse; Murine 17 clone 1 cell line 17Cl1 |
TCID50; RT-qPCR |
|
SARS-CoV-2 Isolate USA-WA1/2020 (BEI or ATCC (BEI NR-52281)) |
Enveloped virus; human beta coronavirus |
Human; Vero cells (ATCC CCL-81) |
TCID50; RT-qPCR |
To date, the microbiostats that have been tested have a single active ingredient on their labels (3- (trihydroxysilyl) propyldimethyloctadecyl ammonium chloride) in varying weight percentages by product. Table 2 lists the products that are currently included in our testing with reported active ingredient concentrations; product names are currently being withheld until additional testing is complete. This update provides initial results for two of the products (Products A and B) applied via electrostatic sprayer (with a nominal 40-micron droplet size) on to pre-cleaned and sterilized 304 stainless steel.
Effective neutralization of the product during the viral analysis is an essential component of the efficacy assessment protocol. A neutralization panel was conducted based upon the volume (approximately 100 µL) of product applied to each coupon via electrostatic sprayer. Table 2 also lists the neutralization methods developed for each product and virus combination (see Table 2). Material types tested include 304 stainless steel, 301 stainless steel, and Acrylonitrile Butadiene Styrene (ABS) plastic.
Table 2: Product information and neutralization methods
|
Products |
Active ingredients |
Neutralization methods |
|---|---|---|
|
Product A |
3- (trihydroxysilyl) propyldimethyloctadecyl ammonium chloride (0.75%) |
MS2/phi6: 10% Dey-Engley broth in PBS |
|
Product B |
3- (trihydroxysilyl) propyldimethyloctadecyl ammonium chloride (<1%) |
MS2/phi6: 10% Dey-Engley broth in PBS |
Current Results
Current testing assessed the recovery of infective phi6 virus from 304 stainless steel test coupons coated with Product A or Product B, compared to recovery from uncoated 304 stainless steel control coupons. Each product was applied to a batch of test coupons using a 10 second application from an electrostatic sprayer operated at a three-foot distance from the coupon surface. Coated coupons dried overnight. The phi6 inoculum contained 5% heat inactivated fetal bovine serum (FBS) in phosphate buffered saline (PBS), in accordance with soil load described in ASTM 1053.
The recoveries of infectious virus on coated and uncoated coupons are reported in Table 3 for each time point post-inoculation. Recoveries of infectious phi6 from coated coupons were similar for both products tested at each time point; differences between products were not statistically significant.
In the draft “Protocol for the Evaluation of Bactericidal Activity of Hard, Non-porous Copper Containing Surface Product”, the performance standard to support registration is a 3-log reduction (99.9%) within a one-hour contact time. Consistent with current registrations for copper containing surface products, EPA intends to revise the protocol to allow up to two hours for the contact time. This contact time and performance standard are currently being considered as a potential performance standard for testing against viruses. The testing reported here does not equate to the entire testing requirements for registration of a product. These results provide an initial screening of products and their potential residual efficacy against viruses.
A 1 to 2 log reduction for coated coupons compared to control coupons was observed for virus-product contact times between zero and 8-hours. At the longer time points (24 and 48 hours contact time), the decay in recoverable infective virus from the coated coupons was similar to that from control coupons (or natural decay of ineffective virus on stainless steel under the ambient laboratory conditions).
Table 3: phi6 recovery from microbiostat coated (electrostatic spray applied) and uncoated
304 stainless steel coupons: 5% FBS in PBS added to the inoculum per ASTM 1053
(note bold rows representing results at the 2 hour contact time)
|
Time |
Mean Recovery |
Standard Deviation |
Efficacy (Log10 Reduction) |
Standard Error |
|---|---|---|---|---|
|
Inoculation Control |
||||
|
0 |
6.21 |
0.04 |
- |
- |
|
Uncoated 304 Stainless Steel |
||||
|
0 |
6.51 |
0.04 |
- |
- |
|
2 |
5.50 |
0.07 |
- |
- |
|
4 |
4.49 |
0.36 |
- |
- |
|
8 |
4.22 |
0.67 |
- |
- |
|
24 |
3.20 |
1.25 |
- |
- |
|
48 |
1.68 |
0.40 |
- |
- |
|
Product A coated 304 Stainless Steel |
||||
|
0 |
6.40 |
0.03 |
0.11 |
0.02 |
|
2 |
4.61 |
0.24 |
0.89 |
0.02 |
|
4 |
3.52 |
0.59 |
0.97 |
0.09 |
|
8 |
2.53 |
0.97 |
1.68 |
0.07 |
|
24 |
2.98 |
0.65 |
0.22 |
0.58 |
|
48 |
1.12 |
0.21 |
0.56 |
0.22 |
|
Product B coated 304 Stainless Steel |
||||
|
0 |
6.23 |
0.04 |
0.29 |
0.02 |
|
2 |
3.96 |
0.24 |
1.54 |
0.02 |
|
4 |
3.34 |
0.53 |
1.15 |
0.12 |
|
8 |
2.86 |
0.41 |
1.35 |
0.33 |
|
24 |
2.22 |
0.34 |
0.99 |
0.68 |
|
48 |
2.24 |
0.21 |
-0.56 |
0.22 |
The testing was also conducted with 10% beef extract in PBS added to the inoculum (instead of 5% FBS), to simulate a more biologically contaminated environment (Table 4). When 10% beef extract was added the inoculum, virus persistence on uncoated control coupons improved and showed minimal decay over the 48 hours. The improved stability resulted in a larger contrast between recoveries from uncoated controls and coated coupons, especially at the longer time points. Again, recoveries of infectious phi6 from coated coupons were similar for both products tested; difference between products was not statistically significant. Log reduction estimates increased from ~1 at 2 hours to > 3 at 48 hours of contact time. Achieving a 3-log reduction required contact times significantly longer than 2 hours discussed above as being considered as one part of a potential performance standard for testing against viruses.
Table 4: phi6 recovery from microbiostat coated (electrostatic spray applied) and uncoated
304 stainless steel coupons: 10% beef extract in PBS added to the inoculum
(note bold rows representing results at the 2 hour contact time)
|
Time |
Mean Recovery |
Standard Deviation |
Efficacy (Log10 Reduction) |
Standard Error |
|---|---|---|---|---|
|
Inoculation Control |
||||
|
0 |
6.42 |
0.09 |
- |
- |
|
Uncoated 304 Stainless Steel |
||||
|
0 |
6.45 |
0.05 |
- |
- |
|
2 |
6.37 |
0.02 |
- |
- |
|
4 |
6.21 |
0.05 |
- |
- |
|
8 |
6.14 |
0.01 |
- |
- |
|
24 |
5.85 |
0.24 |
- |
- |
|
48 |
5.75 |
0.20 |
- |
- |
|
Product A coated 304 Stainless Steel |
||||
|
0 |
6.39 |
0.04 |
0.06 |
0.03 |
|
2 |
5.42 |
0.11 |
0.95 |
0.01 |
|
4 |
4.97 |
0.29 |
1.24 |
0.00 |
|
8 |
4.22 |
0.19 |
1.93 |
0.00 |
|
24 |
4.38 |
0.22 |
1.47 |
0.12 |
|
48 |
2.47 |
1.20 |
3.28 |
0.36 |
|
Product B coated 304 Stainless Steel |
||||
|
0 |
6.37 |
0.05 |
0.09 |
0.03 |
|
2 |
5.64 |
0.05 |
0.74 |
0.01 |
|
4 |
4.65 |
0.05 |
1.56 |
0.03 |
|
8 |
4.87 |
0.35 |
1.28 |
0.03 |
|
24 |
3.72 |
0.32 |
2.12 |
0.10 |
|
48 |
1.94 |
1.01 |
3.81 |
0.23 |
Since the use of 10% beef extract in the inoculum is not a soil load specified in ASTM 1053 or ASTM 2197-17, additional soil loads were tested. The recoveries of infectious phi6 from the controls as a function of time for several different soil loads added to inocula are shown in Figure 1. The intent is to utilize a soil load consistent with standardized tests that minimizes virus decay on the controls and does not interfere with product assessment. Two inoculum soil load formulas from standardized methods (Bovine Serum Albumin (BSA), Tryptone plus Mucin; and 5% Fetal Bovine Serum (FBS)), one soil load formula not described in standardized test (10% Beef Extract), and one condition without inoculum soil load (Phosphate Buffered Saline (PBS)) were evaluated.
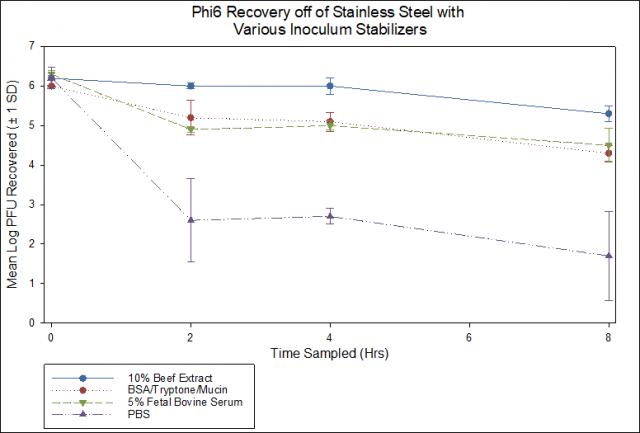
Figure 1: phi6 recovery from control coupons as a function of soil load added to the inoculum
None of the soil loads tested from the standard methods provide the virus stability offered by the inclusion of 10% beef extract. Residual efficacy testing specifies that a product must demonstrate a 3-log reduction in recovered infective virus from test coupons compared to control coupons within a 2-hour time period post-inoculation. Although the 24- and 48-hour contact times in the tests with 10% beef extract as the inoculum soil load showed >3 log reductions for both products tested, this was longer than the 2-hour contact time discussed above as being considered as one part of a potential performance standard for testing against viruses.
At the shorter time scale (up to 8 hours) the difference in recovery from controls as a function of soil load is less pronounced than at the longer (24 and 48 hour) time points. With the emphasis placed on the early time points, testing is moving forward using a soil load from a standard disinfectant efficacy test method (5% FBS in PBS consistent with ASTM 1053). The use of alternatives (e.g., simulated saliva or lung fluid) may be considered in future testing.
The impact of different product application methods is currently being assessed. The application methods include (1) immersion of the coupons in the product for 3 minutes, (2) application of the product with trigger sprayer, and (3) application of the product with a trigger spryer followed by wiping after 3 minutes. These application methods are consistent wth the products’ label instructions.
