Results for SARS-CoV-2 Surface Disinfection with UV-November 10, 2020
Experimental Approach
A standard EPA-approved method does not currently exist for the testing of UV light-generating devices for efficacy (ability to kill or inactivate the target pathogen) against viruses on surfaces. A standardizable approach was developed in this current effort through incorporation of elements of ASTM E3135, Standard Practice for Determining Antimicrobial Efficacy of Ultraviolet Germicidal Irradiation Against Microorganisms on Carriers with Simulated Soil.
This UV research was conducted with SARS-CoV-2 as well as two potential surrogate viruses to SARS-CoV-2, namely bacteriophages MS2 (non-enveloped virus) and Phi6 (enveloped virus) (see Table 1). Results from bacteriophage disinfection studies were used to guide the exposure conditions for the SARS-CoV-2 UV research in a BSL-3 laboratory and support larger-scale and field testing of UV devices.
|
Virus |
Description |
Virus Host |
Analysis Method |
Inoculum |
|---|---|---|---|---|
|
MS2 (ATCC 15597-B1) |
Non-enveloped virus; bacteriophage |
Escherichia coli |
Plaque assay, E. coli C-3000 (ATCC 15597) |
5% Fetal Bovine Serum; spread on surface |
|
Phi6 |
Enveloped virus; bacteriophage |
Pseudomonas syringae |
Plaque assay, P. syringae LM2489 |
5% Fetal Bovine Serum; spread on surface |
|
SARS-CoV-2 Isolate USAWAl/ 2020 (BEI NR-52281) |
Enveloped virus; human beta coronavirus |
Human; Vero cells (ATCC CCL-81) |
TCID50; RT-qPCR on subset |
5% Fetal Bovine Serum and simulated saliva; droplets (no spreading) |
Uniform pieces of surface material (referred to as coupons) were prepared, cleaned, rinsed, and sterilized. The target virus was inoculated onto these coupons. Test coupons were either exposed to UV light immediately (i.e., wet inoculum) or after a time period (typically one hour) to allow the inoculum to dry. Positive controls for each test condition were inoculated on the same material and not exposed to the UV light. All test and positive control coupons were run in triplicate. Negative controls (not inoculated but exposed to UV light) were also included. Material types tested include 304 brushed stainless steel and glass for the MS2 and Phi6 studies and 301 plain stainless steel, ABS plastic, and bus seat fabric for the SARS CoV-2 tests.
UVC-emitting light sources were identified with stakeholder input from Los Angeles County Metropolitan Transportation Authority (LA Metro). They considered a light emitting diode (LED) (Transit Design Group) and a pulsed xenon light (Puro Lighting /Violet Defense) as two UV light emitting devices of interest. While the LED emits a discrete UVC wavelength continuously, the pulsed xenon light source generates a high intensity broad spectrum UV light from 220 nm to 400 nm and into the visible wavelengths with a pulse frequency of one pulse every six seconds. This interim report details research that was conducted with the pulsed xenon light source.
Material coupons were placed in a near-vertical position, with the surface facing the light source at various distances (0.15 m – 2.0 m) and exposed to the light for durations ranging from 3.75 - 60 minutes. For wet inoculations, the light was placed above a table with the material coupons in a horizontal position.
Prior to the start of the bench scale infection testing, LA Metro conducted a field test in an empty metro car to evaluate the feasibility of using the pulse xenon light sources in their nightly routine cleaning and disinfection practices. As part of this field test, LA Metro recorded UV light doses using UV light sensors in selected areas of interest for a variety of test conditions with changing light configurations and exposure durations. Reported UV doses (adjusted to report only the UVC component of the emitted light) were 0.15 to 3.5 mJ/cm2, depending on distance from the sensor to the pulsed xenon light (at a minimum 60 inches), exposure time (30 min or shorter), number of lights present, and whether the sensor was in a direct line of sight or an obscured area.
Current Results November 10, 2020
Bacteriophages Research
Sterile glass and stainless steel coupons (2 cm x 4 cm) were inoculated with either MS2 or Phi6 (each prepared in 5% Fetal Bovine Serum) at a target concentration of ~1 x 106 plaque forming unit (PFU)/coupon. Each coupon was inoculated with a 10 μL droplet which was spread over at least 75% of the coupon surface using the pipette tip. After exposure to UV light, coupons were placed directly in individual tubes containing 10 mL of 10% Dey Engley (DE) broth, which were vortexed for 5 seconds. After completion of all test conditions, virions were extracted from the coupons by an additional vortexing for 2 minutes. Tenfold serial dilutions were prepared for each sample. Each dilution was plated in triplicate using a conventional soft agar overlay method[1] with E. coli and P. syringae as the bacterial hosts for MS2 and Phi6, respectively. After overnight incubation at 35 ± 2° C (E. coli/MS2) or room temperature, 21 ± 2° C (P. syringe/Phi6), plaques were manually enumerated. The efficacy / log reduction was calculated by the difference in mean log10 recoveries of positive control samples (samples not exposed to UV light) and of UV-exposed samples.
Figure 1 shows the observed log reduction of MS2 on 304 stainless steel and glass following UV light exposure from the pulsed xenon light source. Results were collected over multiple days under ambient laboratory conditions with variations in dose (as a function of changing distances and exposure times), material, and inoculation type (wet vs dry inoculum at start of UV exposure). Reported light dose measurements are for emitted light in the 220-280 nm wavelength range (International Light Technologies [ILT] 2500 meter with ILT SED270C sensor) and excludes measurement of UVB and UVA light, which is also emitted from the pulsed xenon source. Recoveries from test coupons were always above the limit of quantification (10 PFU/coupon).
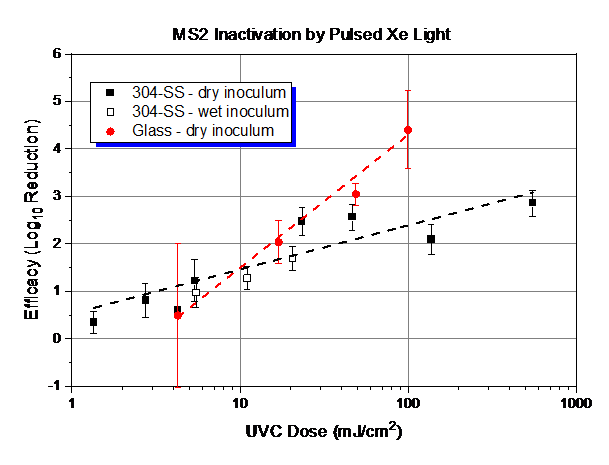
Figure 1: Dose response curve data and linear fits for MS2 and pulsed xenon light. The error bars represent pooled standard deviation from enumeration of both the positive control and test samples. UVC dose measured with ILT 2500 and SED270C sensor.
Results for the Phi6 surrogate are shown in Figure 2 for 304 stainless steel and glass materials following inactivation with the pulsed xenon light source. Recoveries from test coupons were always above the limit of quantification (10 PFU/coupon).
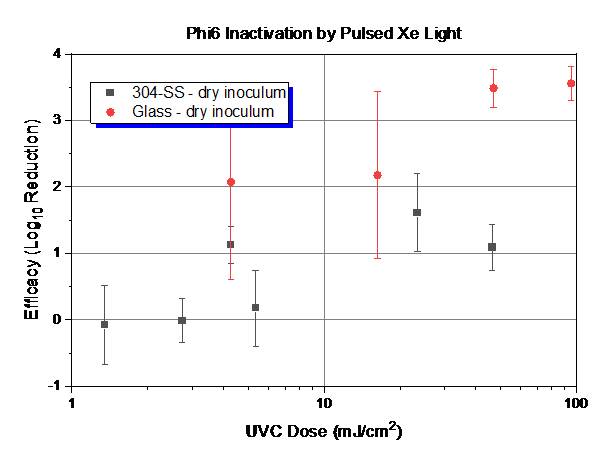
Figure 2: Dose response curve data for Phi6 and pulsed xenon light. The error bars represent pooled standard deviation from enumeration of both the positive control and test samples. UVC dose measured with ILT 2500 and SED270C sensor.
SARS-CoV-2 Virus Research
Sterile ABS plastic, bus seat material (pile: 85% wool, 15% nylon) and 301 stainless steel coupons (1.9 cm x 7.6 cm) were inoculated with SARS-CoV-2 isolate USAWAl/2020 (BEI NR-52281) in storage media (MEM + PenStrep + 5% FBS) or in simulated saliva[2]. Virus was inoculated onto each coupon material, without dilution, as a series of smaller droplets without spreading. A 100 microliter (μL) inoculum was added as 10 by 10 μL droplets. The simulated saliva formulation is a solution designed to mimic the properties of saliva (e.g., pH, protein content, tonicity). Both inoculums had a target concentration of ~2 x 106 Median Tissue Culture Infectious Dose (TCID50) /mL. After exposure to UV light, viable virus was recovered from coupons by adding 10 mL of extraction buffer (e.g., cell culture media) to each tube containing the test samples and associated blanks. The tubes were agitated on an orbital shaker for 15 min at approximately 200 rpm at room temperature. Eluents were tested for viable virus by cell culture (TCID50). Virus concentrations in the eluent were determined and compared to inoculum concentration to determine extraction efficiency. Extraction and recovery of infectious virus was assessed immediately after inoculation and after drying on the surface. The efficacy / log reduction was calculated by the difference in mean log10 recoveries of positive control samples (samples not exposed to UV light) and of UV-exposed samples. Recoveries of SARS-CoV-2 in simulated saliva (only) from coupons that were not exposed to UV light (positive controls) were just above 2 x 103 PFU/coupon which, in combination with the limit of detection (6 PFU/coupon), does not allow for a demonstration of a 3 log reduction.
SARS-CoV-2 inactivation data (single dose measurement reported for multiple materials) with the pulsed xenon light source are summarized in Figure 3. Reported data include log reduction for three materials; for inoculum that was dry or wet at the start of the UV exposure; and for the SARS-CoV-2 virus in media or in simulated saliva. Viable virus was recovered from all test coupons at this dose except for three materials and inoculum conditions as indicated in Figure 3.
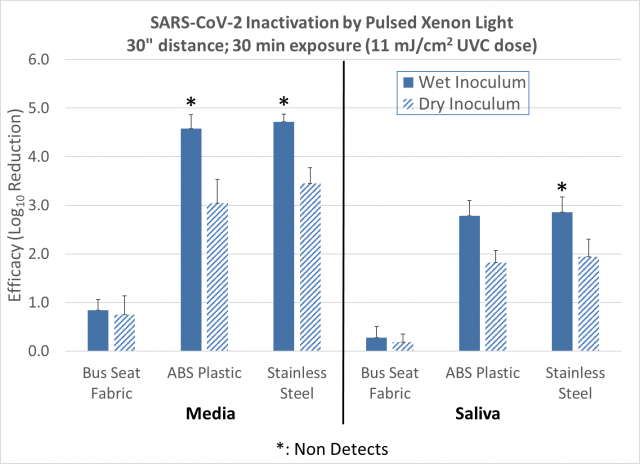
Figure 3: Log reduction of SARS-CoV-2 with pulsed xenon light. The error bars represent pooled standard deviation from enumeration of both the positive control and test samples. Dose information is for multispectral UVC section of emitted UV spectrum. LOQ set at 20 virons/sample. UVC dose measured with ILT 2500 and SED270C sensor.
The applied UVC dose of 11 mJ/cm2 is approximately three times higher than the highest measured dose in the LA Metro car study. Upcoming UVC tests will include a lower UVC dose that was achieved in a metro car.
Comparison of SARS-CoV-2, MS2 and Phi6
A first comparison in measured log reduction by pulsed xenon UV light indicates that SARS-CoV-2 is easier to inactivate than MS2 and Phi6. For the common stainless steel material and a dry inoculum at the start of the UV exposure, the log reduction at a 11 mJ/cm2 dose was 3.5 for SARS-CoV-2 while the log reduction was less than 1.3 for MS2 and Phi6. There are some experimental differences such as the stainless steel appearance / smoothness, inoculum media and inoculum application (droplet versus spread) that require further research as to elucidate their impact on UVC disinfection.
[1] Kropinski, A. M., Mazzocco, A., Waddell, T. E., Lingohr, E., & Johnson, R. P. (2009). Enumeration of bacteriophages by double agar overlay plaque assay. In Bacteriophages (pp. 69-76). Humana Press. Totowa, NJ. doi:10.1007/978-1-60327-164-6_7.
[2] Heimbuch et al. Am. J. Inf. Control 39, 2011; ASTM E2721-16
