September 30 Results for Development of Rapid Viability-Reverse Transcriptase PCR Method
September 30, 2020 Report
Experimental Approach
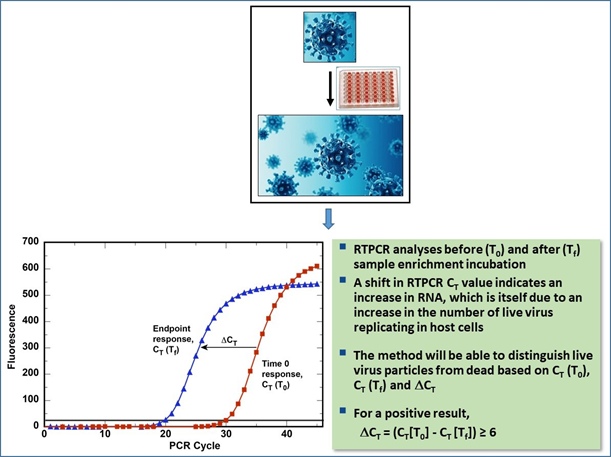
Applying the principle of the RV-PCR methods developed for the above mentioned bacterial biothreat agents, the SARS-CoV-2 RV-RTPCR method integrates cell-culture based enrichment of the virus in a sample with virus-gene-specific RTPCR-based molecular analysis. The RTPCR analysis is conducted before and after the cell-culture-virus (sample) incubation. An optimum algorithm will be established such that the resultant RTPCR cycle threshold (CT) difference (∆CT) between before and after cell-culture-virus incubation RTPCR analyses will determine the presence/absence of viable virus in the sample (Figure 1).
Figure 1. Example Depiction of the SARS-CoV-2 RV-RTPCR Method.
Mouse Hepatitis Virus (MHV) can be handled under Biosafety Level (BSL)-2 conditions, whereas SARS-CoV-2 must be handled at BSL-3 conditions. Both are closely related in the Betacoronavirus genus. Therefore, the RV-RTPCR method logistics have been established efficiently and with fewer restrictions/controls under BSL2 work using MHV and its host cell line, 17C1-1. The MHV effort mainly included selection of appropriate laboratory apparatus format and associated procedures for cell culture and virus infection, and selection of RNA extraction and RTPCR kits. The RV-RTPCR method for MHV was then developed and overall method feasibility was tested. As the next step in a BSL-3 laboratory, the RV-RTPCR method for MHV will be adapted to detect SARS-CoV-2 using the Vero E6 as the host cell line. The method will be further optimized for sensitivity of detection.
Briefly, the MHV/SARS-CoV-2 RV-RTPCR method development includes the following major steps:
- Establish logistics for and develop the RV-RTPCR method using MHV, and its host cell line (17C1-1)
- Establish logistics for RV-RTPCR method development
- Test feasibility of 96-well plate format for the RV-RTPCR method
- Select RNA extraction and RT-PCR kits (in light of unavailability of commonly used kits that are in high demand for clinical sample analysis)
- Evaluate sample (virus) concentration using the Amicon Ultra 15 centrifugal filter device
- Develop the RV-RTPCR method for MHV
- Develop the method using MHV in suspension
- Determine MHV host-cell infection period (time required for the virus to infect host cells)
- Determine post-infection incubation period (time required for virus replication in the host cells after infection) for a good sensitivity of detection
- Evaluate the method performance for swab swatches[1] spiked with MHV
- Adapt the established method logistics and develop the RV-RTPCR method for SARS-CoV-2 using the Vero E6 as the host cell line
- Develop the method using the SARS-CoV-2 suspension
- Determine SARS-CoV-2 host-cell infection period (time required to infect host cells)
- Determine post-infection incubation period (time required for virus replication in the host cells) for a good sensitivity of detection
- Evaluate method performance with SARS-CoV-2-spiked swab swatches
- Evaluate sample (SARS-CoV-2) concentration using the Amicon Ultra 15 centrifugal filter device
- Deliver a Proof-of-Concept RV-RTPCR method for SARS-CoV-2
A minimum of two virus infection periods (e.g., 2-hr and 4-hr or intermediate period) and a minimum of three post-infection incubation periods (e.g., 8-hr, 16-hr, 24-hr or intermediate period) are being evaluated for a short time-to-results with a 101 plaque-forming unit (pfu) level (10 to 99 virus particles) sensitivity of detection. Different dilutions of virus suspension are added in triplicate wells of a 96-well plate for either 2 hr or 4 hr infection, at which time the virus suspension is removed and fresh medium is added. At the proper time (0, 8, 16, or 24 hr post-infection incubation period), the medium is removed from the wells, phosphate buffered saline (PBS) is added, and the plate is frozen at -20°C until RNA extraction is conducted. RTPCR analyses of the RNA extracts are conducted to determine the presence or absence of viable virus in the sample following the algorithm for ∆CT as depicted in Figure 1. For each experiment, the approximate virus titer is also determined from the stock suspension using 50% tissue culture infectious dose (TCID50) analysis. The virus titer for each set of samples is converted to approximate pfu/sample based on the following relationship determined for MHV (Leibowitz et al., 2011[2]): TCID50/sample ´ 0.7 » pfu/sample.
Current Results
The 17C1-1 host cells for the MHV grew well in a 96-well plate format and the optimum cell seeding density was determined for effective virus infection. Therefore, a 96-well plate format was adopted for the RV-RTPCR method development. RNA extraction kits with reported use and efficacy for cell lysis were identified. Due to a delay in getting one of the two RNA extraction kits, the Promega Magnesil Total RNA Mini-isolation System kit was selected. After evaluation of the Superscript III Platinum One-Step qRT-PCR (Invitrogen, Corp.) and One Step PrimeScript III RT-PCR Kit (TakaraBio, Inc.), the TakaraBio One Step PrimeScript III RT-PCR kit was selected for RTPCR. The MHV RTPCR assay targets the replicase gene sequence for the nonstructural protein 2 (nsp2). The Amicon Ultra-15 (10 kDa MWCO) centrifugal filters were tested for virus concentration, and approximately 12.5-fold concentration was achieved.
Using the 96-well format for cell-culture and selected RNA extraction and RTPCR kits, the RV-RTPCR method development for MHV was initiated. Initial experiments were performed using different dilutions of the MHV stock suspension. To determine an optimal virus infection period and post-infection incubation period for MHV with 17Cl-1 cells, 2-hr and 4-hr virus infection periods in combination with 8-, 16-, and 24-hr post-infection incubation periods were evaluated. As shown in Table 1, the 4-hr virus infection period generally had more positive sample replicates than the 2-hr infection period, especially at the lowest virus level, ~1-2 pfu equivalents. In one case for the ~22 pfu/sample level, the 4-hr infection period/8-hr post-infection incubation showed only 1 of 3 positive samples, whereas the 2-hr infection period had 3 of 3 positive samples for this incubation period and virus level. This appeared to be an outlier relative to the other 4-hr infection data and could represent technician error. In general, the results indicated that a 101-pfu (10 to 99 virus particles) sensitivity of detection could be achieved in about 18 hours (2-hr front-end sample processing + 4-hr infection period + 8-hr post-infection incubation period + 4-hr back-end RNA extraction and RTPCR analysis = 18 hours total time-to-results).
Table 1. Summary of RV-RTPCR Results for MHV Suspension Used to Infect
17Cl-1 Cells With 2- and 4-hr Infection – Avg. △CT for Two Replicate Experiments
|
Approx. Virus PFU/Sample* |
Infection Period |
Post-Infection Incubation Period |
Positive Detection |
Avg. DCT ± SD** |
|---|---|---|---|---|
|
~700 |
4 hr |
8 hr |
3 of 3 |
8.2 ± 0.5 |
|
16 hr |
3 of 3 |
14.0 ± 0.4 |
||
|
24 hr |
3 of 3 |
7.6 ± 0.4 |
||
|
2 hr |
8 hr |
3 of 3 |
7.6 ± 0.6 |
|
|
16 hr |
3 of 3 |
12.8 ± 0.8 |
||
|
24 hr |
3 of 3 |
11.5 ± 0.7 |
||
|
~220 |
4 hr |
8 hr |
3 of 3 |
7.3 ± 1.2 |
|
16 hr |
3 of 3 |
16.1 ± 0.9 |
||
|
24 hr |
3 of 3 |
13.7 ± 1.2 |
||
|
2 hr |
8 hr |
3 of 3 |
11.3 ± 2.0 |
|
|
16 hr |
3 of 3 |
20.6 ± 2.0 |
||
|
24 hr |
3 of 3 |
22.3 ± 1.7 |
||
|
~22 |
4 hr |
8 hr |
1 of 3 |
4.2 ± 4.0 |
|
16 hr |
3 of 3 |
21.8 ± 1.6 |
||
|
24 hr |
3 of 3 |
21.0 ± 1.4 |
||
|
2 hr |
8 hr |
3 of 3 |
10.6 ± 0.8 |
|
|
16 hr |
3 of 3 |
17.8 ± 0.3 |
||
|
24 hr |
3 of 3 |
20.5 ± 1.6 |
||
|
~2 |
4 hr |
8 hr |
0 of 3 |
0 |
|
16 hr |
3 of 3 |
17.2 ± 5.3 |
||
|
24 hr |
3 of 3 |
20.9 ± 1.7 |
||
|
2 hr |
8 hr |
0 of 3 |
1.2 ± 1.8 |
|
|
16 hr |
0 of 3 |
0 |
||
|
24 hr |
0 of 3 |
0 |
||
|
~1 |
4 hr |
8 hr |
2 of 3 |
13.2 ± 0.4 |
|
16 hr |
2 of 3 |
21.3 ± 0.9 |
||
|
24 hr |
3 of 3 |
22.6 ± 0.4 |
||
|
2 hr |
8 hr |
2 of 3 |
9.6 ± 2.4 |
|
|
16 hr |
1 of 3 |
5.7 ± 4.6 |
||
|
24 hr |
2 of 3 |
16.3 ± 8.9 |
* Virus titer based on TCID50/sample ´ 0.7 » pfu/sample (Leibowitz et al., 2011)
** This is not a true average DCT and SD since individual CT values are not correlated across the different time points. SD represents the pooled SD which equals the square root of the following: (SD for T0 values squared plus the SD for the Tf values squared)/2, where Tf equals T8, T16, or T24.
Cases for 3 of 3 sample replicates positive are highlighted.
In the above experiments, different dilutions of the neat virus suspension were used to infect host-cells. Since there was no sample processing step to recover virus, there were no virus losses before host cell infection. However, for experiments using a swab or similar sampling tool, the swab will contain different numbers of live virus and there is likely to be virus loss during sample processing. Further, field swab samples would also contain some environmental matrices (e.g. dust, etc.) that could affect virus recovery, in addition to losses during sample processing. Considering these factors as well as the increased sensitivity observed at the lowest virus levels (Table 1), a 4-hr infection period was selected for the experiments with swab swatches. This longer infection time will allow for effective host cell infection to take place, even for a low number of virus particles recovered from the samples, especially in the presence of interferents. For the SARS-CoV-2 RV-RTPCR method, once the experiments with spiked swab swatches are completed using a 4-hr infection period, decreasing infection times of 3 (and possible 2 hrs) will be tested to determine if a shorter post-infection incubation period can be achieved while maintaining the 101 level sensitivity of detection.
Next, the method was evaluated with pre-wetted swab swatches spiked with different dilutions of the MHV stock suspension. A 4-hr virus infection period was used in combination with 8-, 16-, and 24-hr post-infection incubation periods. The virus particles were recovered from the swab swatches, concentrated using the Amicon Ultra-15 centrifugal filters, filtered through 0.22-micron filters, and used for RV-RTPCR analyses. As shown in Table 2, the 4-hr infection period yielded consistent RTPCR Avg. △CT values for positive detection (3 of 3) for all incubation periods for ~80 pfu/sample (based on virus recoveries from TCID50 analysis of replicates swab extracts). The lower number of virus particles (~7 pfu/sample) could also be detected after an 8- or 16-hr post-infection incubation period for 2 of 3 replicates. Therefore, as for experiments with virus suspensions, a 101-pfu level (10 to 99 virus particles) sensitivity of detection was achieved for virus recovered from swab swatches using a 4-hr infection period and 8-hr post-infection incubation period. As mentioned, this would result in an overall infection/post-infection incubation and sample processing/analysis time of about 18 hours.
Table 2. Summary of RV-RTPCR Results for MHV-Spiked Swab Swatches Processed and
Used to Infect 17Cl-1 Cells With 4-hr Infection – Avg. △CT for First Replicate Experiment
|
Approx. Avg. Virus PFU/Sample* |
Infection Period |
Post-Infection Incubation Period |
Positive Detection |
Avg. DCT values |
|---|---|---|---|---|
|
~80 |
4 hr |
8 hr |
3 of 3 |
14.2 ± 2.6 |
|
16 hr |
3 of 3 |
15.5 ± 0.8 |
||
|
24 hr |
3 of 3 |
23.6 ± 0.7 |
||
|
~7 |
4 hr |
8 hr |
2 of 3 |
9.6 ± 2.3 |
|
16 hr |
2 of 3 |
13.8 ± 1.3 |
||
|
24 hr |
1 of 3 |
20.9 |
* Virus titer based on TCID50/sample ´ 0.7 » pfu/sample (Leibowitz et al., 2011).
Cases for 3 of 3 sample replicates positive are highlighted.
Since the MHV RV-RTPCR method yielded a promising sensitivity of detection in about 18 hours for total time-to-results, the RV-RTPCR method development for SARS-CoV-2 detection will be initiated using the same parameters and conditions, except for the use of the Vero E6 host cells and SARS-CoV-2 specific RTPCR assays. Based on the results of such experiments, further method optimization will be performed to increase the sensitivity of detection that will allow for a shorter time-to-results.
[1] Swab swatches were cut to the same dimensions and from the same material as the CDC-recommended swabs (Puritan Cat. No. 25-88060 PF UW), since environment sampling swabs are not available for testing due to current high demand of material for clinical samples.
[2] Leibowitz et al., Current Protocols in Microbiology 15E.1.1-15E.1.46, May 2011.
DOI:10.1002/9780471729259.mc15e01s21
