Basic Ozone Layer Science
The Earth's ozone layerThe region of the stratosphere containing the bulk of atmospheric ozone. The ozone layer lies approximately 15-40 kilometers (10-25 miles) above the Earth's surface, in the stratosphere. Depletion of this layer by ozone depleting substances (ODS) will lead to higher UVB levels, which in turn will cause increased skin cancers and cataracts and potential damage to some marine organisms, plants, and plastics. The science page (http://www.epa.gov/ozone/science/index.html) offers much more detail on the science of ozone depletion. protects all life from the sun's harmful radiation, but human activities have damaged this shield. Less ozone-layer protection from ultraviolet (UV) lightUltraviolet radiation is a portion of the electromagnetic spectrum with wavelengths shorter than visible light. The sun produces UV, which is commonly split into three bands: UVA, UVB, and UVC. UVA is not absorbed by ozone. UVB is mostly absorbed by ozone, although some reaches the Earth. UVC is completely absorbed by ozone and normal oxygen. NASA provides more information on their web site (http://www.nas.nasa.gov/About/Education/Ozone/radiation.html). will, over time, damage crops and lead to higher skin cancer and cataract rates.
I. The Ozone Layer
The Earth's atmosphere is composed of several layers. The lowest layer, the troposphereThe region of the atmosphere closest to the Earth. The troposphere extends from the surface up to about 10 km in altitude, although this height varies with latitude. Almost all weather takes place in the troposphere. Mt. Everest, the highest mountain on Earth, is only 8.8 km high. Temperatures decrease with altitude in the troposphere. As warm air rises, it cools, falling back to Earth. This process, known as convection, means there are huge air movements that mix the troposphere very efficiently., extends from the Earth's surface up to about 6 miles or 10 kilometers (km) in altitude. Virtually all human activities occur in the troposphere. Mt. Everest, the tallest mountain on the planet, is only about 5.6 miles (9 km) high. The next layer, the stratosphereThe region of the atmosphere above the troposphere. The stratosphere extends from about 10km to about 50km in altitude. Commercial airlines fly in the lower stratosphere. The stratosphere gets warmer at higher altitudes. In fact, this warming is caused by ozone absorbing ultraviolet radiation. Warm air remains in the upper stratosphere, and cool air remains lower, so there is much less vertical mixing in this region than in the troposphere., continues from 6 miles (10 km) to about 31 miles (50 km). Most commercial airplanes fly in the lower part of the stratosphere.
Most atmospheric ozone is concentrated in a layer in the stratosphere, about 9 to 18 miles (15 to 30 km) above the Earth's surface (see the figure). Ozone is a molecule that contains three oxygen atoms. At any given time, ozone molecules are constantly formed and destroyed in the stratosphere. The total amount has remained relatively stable during the decades that it has been measured.
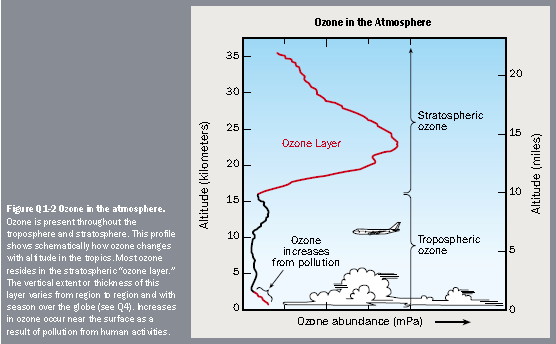
The ozone layer in the stratosphere absorbs a portion of the radiation from the sun, preventing it from reaching the planet's surface. Most importantly, it absorbs the portion of UV light called UVBA band of ultraviolet radiation with wavelengths from 280-320 nanometers produced by the Sun. UVB is a kind of ultraviolet light from the sun (and sun lamps) that has several harmful effects. UVB is particularly effective at damaging DNA. It is a cause of melanoma and other types of skin cancer. It has also been linked to damage to some materials, crops, and marine organisms. The ozone layer protects the Earth against most UVB coming from the sun. It is always important to protect oneself against UVB, even in the absence of ozone depletion, by wearing hats, sunglasses, and sunscreen. However, these precautions will become more important as ozone depletion worsens. NASA provides more information on their web site (http://www.nas.nasa.gov/About/Education/Ozone/radiation.html).. UVB has been linked to many harmful effects, including skin cancers, cataracts, and harm to some crops and marine life.
Scientists have established records spanning several decades that detail normal ozone levels during natural cycles. Ozone concentrations in the atmosphere vary naturally with sunspots, seasons, and latitude. These processes are well understood and predictable. Each natural reduction in ozone levels has been followed by a recovery. Beginning in the 1970s, however, scientific evidence showed that the ozone shield was being depleted well beyond natural processes.
II. Ozone Depletion
When chlorine and bromine atoms come into contact with ozone in the stratosphere, they destroy ozone molecules. One chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. Ozone can be destroyed more quickly than it is naturally created.
Some compounds release chlorine or bromine when they are exposed to intense UV light in the stratosphere. These compounds contribute to ozone depletion, and are called ozone-depleting substances (ODSA compound that contributes to stratospheric ozone depletion. ODS include chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), halons, methyl bromide, carbon tetrachloride, hydrobromofluorocarbons, chlorobromomethane, and methyl chloroform. ODS are generally very stable in the troposphere and only degrade under intense ultraviolet light in the stratosphere. When they break down, they release chlorine or bromine atoms, which then deplete ozone. A detailed list (http://www.epa.gov/ozone/science/ods/index.html) of class I and class II substances with their ODPs, GWPs, and CAS numbers are available.). ODS that release chlorine include chlorofluorocarbonsGases covered under the 1987 Montreal Protocol and used for refrigeration, air conditioning, packaging, insulation, solvents, or aerosol propellants. Since they are not destroyed in the lower atmosphere, CFCs drift into the upper atmosphere where, given suitable conditions, they break down ozone. These gases are being replaced by other compounds: hydrochlorofluorocarbons, an interim replacement for CFCs that are also covered under the Montreal Protocol, and hydrofluorocarbons, which are covered under the Kyoto Protocol. All these substances are also greenhouse gases. See hydrochlorofluorocarbons, hydrofluorocarbons, perfluorocarbons, ozone depleting substance. (CFCs), hydrochlorofluorocarbonsCompounds containing hydrogen, fluorine, chlorine, and carbon atoms. Although ozone depleting substances, they are less potent at destroying stratospheric ozone than chlorofluorocarbons (CFCs). They have been introduced as temporary replacements for CFCs and are also greenhouse gases. See ozone depleting substance. (HCFCs), carbon tetrachlorideA compound consisting of one carbon atom and four chlorine atoms. Carbon tetrachloride was widely used as a raw material in many industrial uses, including the production of chlorofluorocarbons (CFCs), and as a solvent. Solvent use ended when it was discovered to be carcinogenic. It is also used as a catalyst to deliver chlorine ions to certain processes. Its ozone depletion potential is 1.2., and methyl chloroformA compound consisting of carbon, hydrogen, and chlorine. Methyl chloroform is used as an industrial solvent. Its ozone depletion potential is 0.11.. ODS that release bromine include halonsCompounds, also known as bromofluorocarbons, that contain bromine, fluorine, and carbon. They are generally used as fire extinguishing agents and cause ozone depletion. Bromine is many times more effective at destroying stratospheric ozone than chlorine. See ozone depleting substance. and methyl bromideA compound consisting of carbon, hydrogen, and bromine. Methyl Bromide is an effective pesticide used to fumigate soil and many agricultural products. Because it contains bromine, it depletes stratospheric ozone and has an ozone depletion potential of 0.6. Production of methyl bromide was phased out on December 31, 2004, except for allowable exemptions. Much more information is available (http://www.epa.gov/ozone/mbr/index.html).. Although ODS are emitted at the Earth’s surface, they are eventually carried into the stratosphere in a process that can take as long as two to five years.
In the 1970s, concerns about the effects of ozone-depleting substances (ODSA compound that contributes to stratospheric ozone depletion. ODS include chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), halons, methyl bromide, carbon tetrachloride, hydrobromofluorocarbons, chlorobromomethane, and methyl chloroform. ODS are generally very stable in the troposphere and only degrade under intense ultraviolet light in the stratosphere. When they break down, they release chlorine or bromine atoms, which then deplete ozone. A detailed list (http://www.epa.gov/ozone/science/ods/index.html) of class I and class II substances with their ODPs, GWPs, and CAS numbers are available.) on the stratospheric ozone layerThe region of the stratosphere containing the bulk of atmospheric ozone. The ozone layer lies approximately 15-40 kilometers (10-25 miles) above the Earth's surface, in the stratosphere. Depletion of this layer by ozone depleting substances (ODS) will lead to higher UVB levels, which in turn will cause increased skin cancers and cataracts and potential damage to some marine organisms, plants, and plastics. The science page (http://www.epa.gov/ozone/science/index.html) offers much more detail on the science of ozone depletion. prompted several countries, including the United States, to ban the use of chlorofluorocarbons (CFCsOrganic compounds made up of atoms of carbon, chlorine, and fluorine. An example is CFC-12 (CCI2F2), used as a refrigerant in refrigerators and air conditioners and as a foam blowing agent. Gaseous CFCs can deplete the ozone layer when they slowly rise into the stratosphere, are broken down by strong ultraviolet radiation, release chlorine atoms, and then react with ozone molecules. See Ozone Depleting Substance.) as aerosolA small droplet or particle suspended in the atmosphere, typically containing sulfur. Aerosols are emitted naturally (e.g., in volcanic eruptions) and as the result of human activities (e.g., by burning fossil fuels). There is no connection between particulate aerosols and pressurized products also called aerosols. (See below) propellants. However, global production of CFCs and other ODS continued to grow rapidly as new uses were found for these chemicals in refrigeration, fire suppression, foam insulation, and other applications.
Some natural processes, such as large volcanic eruptions, can have an indirect effect on ozone levels. For example, Mt. Pinatubo's 1991 eruption did not increase stratospheric chlorine concentrations, but it did produce large amounts of tiny particles called aerosolsSmall particles or liquid droplets in the atmosphere that can absorb or reflect sunlight depending on their composition. (different from consumer products also known as aerosols). These aerosols increase chlorine's effectiveness at destroying ozone. The aerosols in the stratosphere create a surface on which CFC-based chlorine can destroy ozone. However, the effect from volcanoes is short-lived.
Not all chlorine and bromine sources contribute to ozone layer depletion. For example, researchers have found that chlorine from swimming pools, industrial plants, sea salt, and volcanoes does not reach the stratosphere. In contrast, ODS are very stable and do not dissolve in rain. Thus, there are no natural processes that remove the ODS from the lower atmosphere.
One example of ozone depletion is the annual ozone "hole" over Antarctica that has occurred during the Antarctic spring since the early 1980s. This is not really a hole through the ozone layer, but rather a large area of the stratosphere with extremely low amounts of ozone.
Ozone depletion is not limited to the area over the South Pole. Research has shown that ozone depletion occurs over the latitudes that include North America, Europe, Asia, and much of Africa, Australia, and South America. More information about the global extent of ozone depletion can be found in the Scientific Assessment of Ozone Depletion: 2018 developed by the United Nations Environment Programme.
