Basic Information About Provisional Peer-Reviewed Toxicity Values (PPRTVs)
On this page:
Learn About Provisional Peer-Reviewed Toxicity Values
1. What are PPRTVs?
EPA’s PPRTV Program supports the Agency’s mission to protect human health and the environment by identifying and characterizing the health hazards of, and providing an important source of toxicity information and toxicity values for, chemicals of concern to the Superfund Program.
PPRTV assessments are developed in response to requests from EPA’s Superfund Program to the Superfund Health Risk Technical Support Center (STSC) within EPA’s Office of Research and Development’s (ORD’s) Center for Public Health and Environmental Assessment (CPHEA). PPRTVs are derived after a review of the relevant scientific literature and using Agency methodologies, practices, and guidance for the development of toxicity values (e.g., oral reference doses (RfDs), inhalation reference concentrations (RfCs), provisional oral slope factors (p-OSF), and provisional inhalation unit risks (p-IUR)).
All PPRTV assessments receive internal review by EPA scientists and external peer review by independent scientific experts. For additional information on the methodologies used, please refer to "Guidance & Tools". PPRTV assessments are eligible to be updated as requested by the Agency to incorporate new data or methodologies that might impact the science and decisions used to derive provisional toxicity values, and are revised as appropriate.
2. Who develops PPRTVs?
PPRTVs are assessments developed by scientists in ORD’s Center for Public Health and Environmental Assessment (CPHEA) who are recognized internationally for their expertise in toxicology, epidemiology, biology, chemistry, and statistics.
- Learn more about the Center for Public Health and Environmental Assessment
- Learn more about the Office of Research and Development
3. What types of PPRTVs are derived?
PPRTV assessments provide the following provisional toxicity values for health effects resulting from chronicUsed to describe recurring symptoms or disease. or subchronic exposureRepeated exposure by the oral, dermal, or inhalation route for more than 30 days, up to approximately 10% of the life span in humans (more than 30 days up to approximately 90 days in typically used laboratory animal species). [See also longer-term exposure.] to chemicals, they are typically grouped as noncancer assessments and cancer assessments.
Noncancer Assessments
The following terms describe the types of values that are derived:
Provisional Reference Dose (p-RfD): An estimate (with uncertainty spanning perhaps an order of magnitude) of a daily oral (ingested) exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious health effects during a lifetime. It can be derived from a NOAELThe highest exposure level at which there are no biologically significant increases in the frequency or severity of adverse effect between the exposed population and its appropriate control; some effects may be produced at this level, but they are not considered adverse or precursors of adverse effects. (no observed adverse effect level), LOAELThe lowest exposure level at which there are biologically significant increases in frequency or severity of adverse effects between the exposed population and its appropriate control group. (lowest observed adverse effect level), or benchmark dose, with uncertainty factors generally applied to reflect limitations of the data used.
Provisional Reference Concentration (p-RfC): An estimate (with uncertainty spanning perhaps an order of magnitude) of a continuous inhalation exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious health effects during a lifetime. It can be derived from a NOAEL, LOAEL, or benchmark concentration, with uncertainty factors generally applied to reflect limitations of the data used.
RfCAn estimate (with uncertainty spanning perhaps an order of magnitude) of a continuous inhalation exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime. It can be derived from a NOAEL, LOAEL, or benchmark concentration, with uncertainty factors generally applied to reflect limitations of the data used. Generally used in EPA's noncancer health assessments. [Durations include acute, short-term, subchronic, and chronic and are defined individually in this glossary]. Cancer Assessments
Cancer descriptors characterize the chemical as:
- Carcinogenic to Humans
- Likely to Be Carcinogenic to Humans
- Suggestive Evidence of Carcinogenic Potential
- Inadequate Information to Assess Carcinogenic Potential
- Not Likely to Be Carcinogenic to Humans
The following terms describe the types of values that are derived:
Provisional oral slope factor An upper bound, approximating a 95% confidence limit, on the increased cancer risk from a lifetime oral exposure to an agent. This estimate, usually expressed in units of proportion (of a population) affected per mg/kg-day, is generally reserved for use in the low-dose region of the dose-response relationship, that is, for exposures corresponding to risks less than 1 in 100. (p-OSF): An estimate of the increased cancer risk from oral exposure to a dose of 1 mg/kg-day for a lifetime. The OSF can be multiplied by an estimate of lifetime exposure (in mg/kg-day) to estimate the lifetime cancer risk.
Provisional inhalation unit riskThe upper-bound excess lifetime cancer risk estimated to result from continuous exposure to an agent at a concentration of 1 µg/L in water, or 1 µg/m³ in air. The interpretation of unit risk would be as follows: if unit risk = 2 × 10⁻⁶ per µg/L, 2 excess cancer cases (upper bound estimate) are expected to develop per 1,000,000 people if exposed daily for a lifetime to 1 µg of the chemical per liter of drinking water. (p-IUR): An estimate of the increased cancer risk from inhalation exposure to a concentration of 1 µg/m3 for a lifetime. The IUR can be multiplied by an estimate of lifetime exposure (in µg/m3) to estimate the lifetime cancer risk.
Screening Assessments
Screening values are derived when the data do not meet all requirements for deriving a provisional toxicity value. They can be screening subchronic and chronic p-RfDs and p-RfCs, as well as screening p-OSFs and p-IURs. Screening PPRTVs are derived using the same methodologies and undergo the same development and review processes (i.e., internal and external peer review, etc.) as provisional values; however, the screening values are presented in an appendix and characterized such that users of screening PPRTVs are made aware that there is more uncertainty associated with these screening values than for the values presented in the main body of a PPRTV assessment.
4. Under what circumstances are screening PPRTVs derived?
When some useful human or animal toxicity data are available for a chemical, but…
- The data are published in non peer-reviewed sources.
- The data are published and peer-reviewed, but have associated uncertainties such as:
- The composite Uncertainty Factor is greater than 3,000.
- The principal study is not comprehensive (e.g., few or one endpoint examined).
- Other: the principal study has a small number of animals tested, poor study design, incomplete reporting, etc.
- When no useful human or animal toxicity data are available for a chemical…
- An expert-driven read-across approach can be applied.
5. What is an expert-driven read across approach?
An expert-driven read across approach is defined as:
- A framework designed to address data gaps for chemicals with limited in vivo toxicity information for quantitative human health risk assessment (for additional information, see journal article by Wang et al., 2012).
- Relies on the identification of three main types of potential analogues (structural, metabolic/toxicokinetic, and toxicity).
- A weight-of-evidence (WoE) approach is applied for the selection of a final/best surrogate chemical based on structural, metabolic/toxicokinetic, and toxicity similarity.
- The point of departure (POD) for the chosen surrogate is used to derive screening PPRTVs for the target chemical of concern
Guidance & Tools
EPA follows Agency guidance in developing PPRTV assessments. Key guidelines, technical documents and a EPA tools used by the PPRTV Program for developing assessments are listed below. Additional Agency guidance, models and tools are available at EPA's Risk Assessment website.
EPA Guidance Documents
Includes:
- Guidance Documents
- Tools
EPA Guidance Documents
EPA Cancer Guidelines
- U.S. EPA. 2005. Guidelines for Carcinogen Risk Assessment EPA/630/P-03/001F, Mar 2005.
- U.S. EPA. 2005. Supplemental Guidance for Assessing Susceptibility from Early-Life Exposure to Carcinogens EPA/630/R-03/003F, Mar 2005.
EPA Risk Assessment Guidelines
- U.S. EPA. 2012. Guideline for Microbial Risk Assessment: Pathogenic Microorganisms with Focus on Food and Water. EPA/100/J-12/001, Jul 2012.
- U.S. EPA. 2000. Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures. EPA/630/R-00/002, Aug 2000.
- U.S. EPA. 1998. Guidelines for Neurotoxicity Risk Assessment EPA/630/R-95/001F, Apr 1998.
- U.S. EPA, 1996. Guidelines for Reproductive Toxicity Risk Assessment EPA/630/R-96/009, Oct 1996.
- U.S. EPA. 1991. Guidelines for Developmental Toxicity Risk Assessment EPA/600/FR-91/001, Dec 1991.
- U.S. EPA. 1986. Guidelines for Mutagenicity Risk Assessment EPA/630/R-98/003, Sep 1986.
- U.S. EPA. 1986. Guidelines for the Health Risk Assessment of Chemical Mixtures EPA/630/R-98/002, Sep 1986.
EPA Science Policy Council Guidelines
- U.S. EPA. 2015. Science Policy Council Handbook: Peer Review. Fourth Edition. Office of Science Policy, Office of Research and Development, Washington, DC. EPA/100/B-15/001, Oct 2015.
- U.S. EPA. 2000. Science Policy Council Handbook: Risk Characterization. Office of Science Policy, Office of Research and Development, Washington, DC. EPA 100-B-00-002, Dec 2000.
Other Guidance Documents and Technical Panel Reports
- U.S. EPA. 2014. Guidance for Applying Quantitative Data to Develop Data-Derived Extrapolation Factors for Interspecies and Intraspecies Extrapolation. EPA/100/R-14/022F, Sep 2014.
- U.S. EPA. 2014. Framework for Human Health Risk Assessment to Inform Decision Making. EPA/100/R-14/001, Apr 2014.
- U.S. EPA. 2012. Advances in Inhalation Gas Dosimetry for Derivation of a Reference Concentration (RfC) and Use in Risk Assessment. EPA/600/R-12/044, Sep 2012.
- U.S. EPA. 2012. Benchmark Dose Technical Guidance. EPA/100/R-12/001, Jun 2012.
- U.S. EPA. 2011. Recommended Use of Body Weight 3/4 as the Default Method in Derivation of the Oral Reference Dose. EPA/100/R11/0001, Feb 2011.
- U.S. EPA. 2006. Approaches for the Application of Physiologically Based Pharmacokinetic (PBPK) Models and Supporting Data in Risk Assessment. EPA/600/R-05/043F, Sep 2006.
- U.S. EPA. 2006. A Framework for Assessing Health Risks of Environmental Exposure to Children. EPA/600/R-05/093F, Sep 2006.
- U.S. EPA. 2002. A Review of the Reference Dose and Reference Concentration Processes. EPA/630/P-02/002F, Dec 2002.
- U.S. EPA. 1994. Methods for Derivation of Inhalation Reference Concentrations and Application of Inhalation Dosimetry. EPA/600/8-90/066F, Oct 1994.
- U.S. EPA. 1988. Recommendations for and Documentation of Biological Values for Use in Risk Assessment. EPA 600/6-87/008, Feb 1988.
References Cited in Older Assessment Documents but Superseded by More Recent Guidance
- U.S. EPA. 2006. Science Policy Council Handbook: Peer Review. Third Edition. Office of Science Policy, Office of Research and Development, Washington, DC. EPA/100/B-06/002, Jan 2006.
- U.S. EPA. 2000. Science Policy Council Handbook: Peer Review. Second Edition. Office of Science Policy, Office of Research and Development, Washington, DC. EPA EPA/100/B-00/001
- U.S. EPA. 2000. Benchmark Dose Technical Guidance Document External Review Draft. EPA/630/R-00/001, Oct 2000.
- U.S. EPA. 1999. Guidelines for Carcinogen Risk Assessment Review draft. NCEA-F-0644, Jul 1999.
- U.S. EPA. 1996. Proposed Guidelines for Carcinogen Risk Assessment. EPA/600/P-92/003C, Apr 1996.
- U.S. EPA. 1993. Reference Dose (RfD): Description and Use in Health Risk Assessments, Mar 1993.
- U.S. EPA. 1992. EPA's Approach for Assessing the Risks Associated with Chronic Exposures to Carcinogens, Jan 1992.
- U.S. EPA. 1986. Risk Assessment Guidelines of 1986. EPA/600/8-87/045, Sep 1987.
- U.S. EPA. 1986. Guidelines for Carcinogen Risk Assessment. EPA/630/R-00/004, Sep 1986.
Relevant Scientific Journal Articles
Tools
Benchmark Dose Software (BMDS)
Benchmark dose (BMD) modeling is EPA’s preferred approach for deriving points of departure (PODs) used to develop toxicity values in PPRTV assessments. Use of BMD modeling involves fitting a set of mathematical models to dose-response data from human and animal studies. EPA’s benchmark dose software (BMDS) was designed to facilitate the application of BMD methods in dose-response assessment.
Health and Environmental Research Online (HERO)
HERO is a searchable database of more than 1.6 million scientific studies and other references used to support the development of EPA assessments like the Provisional Peer-Review Toxicity Value (PPRTV) assessments. Each HERO record provides detailed bibliographic information with associated project pages for some of the more recent PPRTV assessments.
Integrated Risk Information System(IRIS)
IRIS is a searchable database of more than 500+ chemical assessments and other references used to support the development of EPA assessments. Each IRIS chemical landing page provides the available toxicity values, assessment status details, and a history of supporting documents. The IRIS database is used as a reference for the application and practice of current risk assessment methodologies to ensure consistency across PPRTV assessments and other CPHEA products.
Regional Screening Levels (RSLs)
The RSL tables provide comparison values for residential and commercial/industrial exposures to soil, air, and tapwater (drinking water). The unified use of the RSLs, to screen chemicals at Superfund sites, promotes national consistency. The RSLs tables include risk-based screening levels, calculated using the latest toxicity values, default exposure assumptions and physical and chemical properties, and a calculator where default parameters can be changed to reflect site-specific risks. PPRTV assessments are a significant source of toxicity values for the RSL tables.
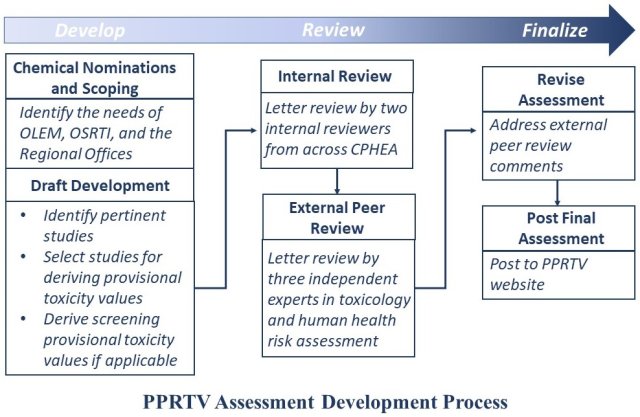
PPRTV Process for Developing Assessments
Before beginning to develop a draft PPRTV assessment in response to the Superfund Program’s request, the PPRTV Program undertakes an internal chemical scoping process to characterize the needs for an assessment. A Scoping team screens available data and prioritizes chemicals on an annual basis to develop the list for PPRTV development.
- Two CPHEA scientists internally review the draft PPRTV assessment, and it is revised based on the comments received.
- The draft PPRTV assessment undergoes a letter external peer review by three independent experts in toxicology and human health risk assessment.
- The PPRTV Program revises the assessment to address external peer review comments. They also prepare a written response-to-comment document.
- The final PPRTV assessment is posted to the PPRTV website.
Note: To learn more about the historical development of the PPRTV Process see the "history of PPRTVs".
History of the Provisional Peer-Reviewed Toxicity Values Program
Important milestones include:
- 2018 - EPA migrated the PPRTV website and database from the Oak Ridge National Laboratory (ORNL) server to the EPA website.
- 2008 - EPA began developing screening PPRTVs for chemicals when the data do not meet all requirements for deriving a toxicity value.
- 2004 - EPA's PPRTV assessments were made available to Superfund sites and risk assessors via a limited accessibility website managed by ORNL.
- 1998 - EPA began developing PPRTV assessments to support a growing need for toxicity values for the Superfund Program. Within the same year, the Superfund Health Risk Technical Support Center (STSC) was also established to provide the resources needed to manage the PPRTV development process.