Adverse Outcome Pathway (AOP) Research Brief
- Overview
- AOP Concepts
- Key AOP Principles
- How AOPs are Used
- Considerations When Using AOPs
- Additional Resources
Overview
Many chemical and nonchemical “stressors” in the environment are potential hazards that can harm living things. The severity of a harmful health effect, or adverse outcome, depends on the timing and level of exposure. Adverse outcome pathways (AOPs) are a conceptual framework, or a way of organizing data into documents, that is used to assess health hazards to humans or ecological species such as fish or honeybees.
Traditional toxicity tests typically measure observable health effects, or adverse outcomes, in animals following exposure to different levels of a single chemical. These tests in animals are called in vivo studies. AOPs help researchers use animal-free in vitro screening data to predict which chemicals may cause adverse effects. AOPs can help identify which chemicals in the environment may cause adverse outcomes, such as cancer, that are relevant to risk assessment and regulatory decision making. This identification can help prioritize chemicals for research purposes.
Prioritization is important because only a small percentage of the many thousands of chemicals that are already present in the environment have been studied using traditional toxicity tests. This approach helps narrow down the list of chemicals, prioritizing them for subsequent testing to answer specific questions about the chemical hazard, such as “What level of exposure causes harm?” Thus, the AOP framework supports the use of different types of biological data to complement or potentially replace in vivo animal studies.
AOP Concepts
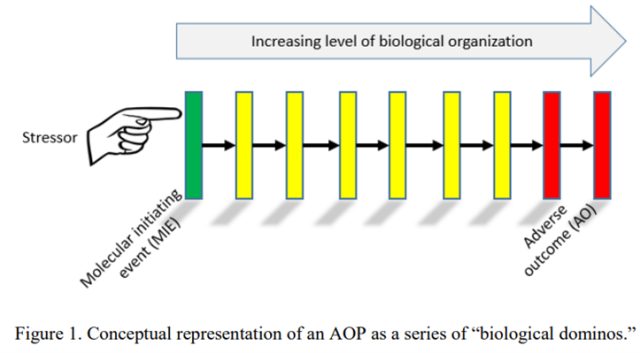
An AOP is like a series of “biological dominos” (Figure 1). A stressor triggers a reversible or irreversible biological change through a direct interaction with molecules in the body (e.g., a chemical binding to DNA). This interaction is called a molecular initiating event (MIE). The MIE represents the first biological domino in the sequence.
If the MIE is severe enough, it can cause additional biological dominos to fall, where each domino represents a key event (KE) at increasing levels of biological organization (cells à tissues à organs) or within other parts of the body (e.g., DNA damage inside a cell leads to tumor formation in liver tissue).
Each KE can be measured (i.e., one can “see” the domino fall) to track progression towards the adverse outcome, which is a health effect such as tumor formation that is considered relevant for risk assessment or regulatory decision making. Each KE in the sequence is viewed as “essential”, meaning if the KE is not observed (i.e., the domino does not fall), then none of the downstream KEs in the pathway will occur (i.e., no additional dominos in the sequence will fall).
Key event relationships (arrows in Figure 1) describe how a particular biological change, represented as a KE, will trigger the next KE in the sequence (i.e., cause the next domino to fall). KERs are defined based on three types of evidence:
- Biological plausibility – what is known about the biology supports the relationship
- Empirical support – experimental evidence that KE1 causes KE2
- Quantitative understanding – the conditions (e.g., timing, magnitude, or duration) under which a change in KE1 will cause a change in KE2
KERs help predict the likelihood that downstream biological dominos will fall based on observation of one or more upstream dominos. A clear description of the evidence supporting each KER determines the level of confidence in each step along the pathway. This helps determine the types of risk assessment or regulatory decisions the AOP can be used to support.
Key AOP Principles
- AOPs are not stressor specific: AOPs depict a generalized sequence of biological effects that can be expected for any stressor that directly changes a particular biological target (defined by the MIE). For example, several different chemicals could all trigger the same MIE for a given AOP.
- AOPs are modular: Any AOP can be represented as a sequence of “nodes” (KEs) and “edges” (KERs) linking those KEs together (Figure 2). KEs represent assays and endpoints (effects) that can be measured or observed, while KERs provide the basis for linking one KE to the next.
- AOPs are a tool used to evaluate biological effects: Individual AOPs are a deliberate simplification of complex biological systems intended to support regulatory decision making and help identify uncertainties that can be a focus of additional testing.
- AOP networks are the functional unit of prediction: Multiple AOPs sharing common KEs (nodes) and/or KERs (links) can be assembled into networks . AOP networks capture complexity of real biological systems and become more complete as more AOPs are defined.
- AOPs are living documents: AOPs are primarily a way of organizing information. As new evidence and understanding supporting KERs and/or new methods for measuring KEs emerge, AOPs can be continually expanded or refined.
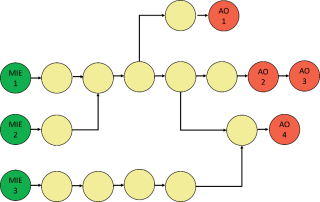
Figure 2: An AOP network consists of multiple AOPs linked by shared KE “nodes” (circles) that are connected by KER “edges” (arrows). This figure shows an AOP network of seven AOPs. Each AOP starts with a MIE and ends with an adverse outcome. The same MIE or adverse outcome can be part of multiple AOPs.
How AOPs are Used
The AOP framework is a translational tool that is used to enhance communication between scientists who generate toxicity data and the potential end users of this information, such as risk assessors or decision makers. AOPs are built using information that is documented in a consistent and organized manner to make it easier for decision makers to access and understand complex datasets.
AOPs are used to address the challenge of assessing and managing human health and ecological risks associated with the tens of thousands of chemicals entering or present in the environment. There are several areas in which a pathway-based understanding of chemical effects can substantially enhance chemical safety assessment:
- Enhanced use of data from new approach methods: When data from traditional in vivo animal studies are lacking for a given chemical, in vitro experiments may provide insights into the chemical’s hazard potential if there is an AOP that links the in vitro data to an adverse outcome. For example, a chemical causes a specific DNA mutation in an in vitro screening assay. That DNA mutation is the MIE in an AOP for liver cancer (the adverse outcome). The information in the AOP could be used as one of the tools to assess whether the chemical is a potential carcinogen.
- Evaluation of uncertainty: AOP descriptions provide qualitative strength of evidence linking each pair of KEs in the pathway. This allows the decision maker to compare the confidence in the AOP with the chemical-specific data available for a given risk assessment and decide on the suitability of that AOP for supporting different types of decisions. For example, there is robust evidence linking reduced maternal thyroid hormone levels (KE) with adverse developmental outcomes. This evidence supports the regulation of chemicals for which exposures result in reduced thyroid hormone levels.
- Hypothesis-driven testing: Knowledge of health effects likely to follow a given MIE can help focus in vivo testing that may need to be done for a chemical to identify sensitive species, life-stages, and toxicity endpoints. For example, estrogen mimicking chemicals (i.e., chemicals with structures that look like estrogen) can bind the estrogen receptor (MIE) and lead to changes in sex ratios in a fish population (adverse outcome). Therefore, exposure studies using surrogate fish species are useful to identify the presence of estrogen disrupting chemicals in waterways.
- Cross-species extrapolation: An uncertainty in both human health and ecological risk assessment involves the extrapolation of toxicity data from tested to untested species. The use of AOP knowledge to directly evaluate conservation of pathways and quantitative differences in toxicological response across species can help address this challenge. For example, if a fish species used in toxicity testing and an untested endangered fish species have structurally conserved estrogen receptors (confirmed using EPA’s SeqAPASS tool), then an AOP linking estrogen receptor activation (MIE) to disrupted sex ratios (adverse outcome) in the test species would support the extrapolation that biologically active concentrations of the chemical will also disrupt sex ratios in the untested fish species.
- Evaluation of complex mixtures: An additional challenge in chemical risk assessment involves understanding the toxicity of new or existing chemical mixtures. Insights provided by AOP networks can help address uncertainties associated with prediction of mixture effects. For example, the insight that two chemicals share a KE (e.g., reduced maternal thyroid hormone levels) leading to the same adverse outcome (e.g., reduced birthweight) can inform in vivo experiments to test the hypothesis that they have a dose additive effect.
Considerations When Using AOPs
- AOPs are not risk assessments: AOPs inform the characterization of hazard or effect; they do not explicitly address exposure, which is a key component of a risk assessment.
- AOPs are not synonymous with high-throughput testing or pathway-based assays: AOPs are assemblies of knowledge designed to aid the interpretation of high-throughput testing or pathway-based data in the context of relevant health effects. However, they do not represent actual biological assays.
- AOPs are not mode of action analyses: The mode of action framework, as applied in human health risk assessment, represents a systematic description and analysis of how a specific chemical causes an adverse effect in an organism. AOPs, which are intended to be generalizable to any chemical acting on a particular MIE, can be applied in mode of action analysis, but the terms are not synonymous.
- AOPs are not computational models: AOPs facilitate a quantitative understanding of how alteration in one KE impacts downstream KEs. While this information may be represented in the form of one or more computational models, AOPs are not standalone computational models.
- AOPs are not a “silver bullet”: AOPs do not describe every detail of adverse and adaptive biology underlying an organism’s response to a stressor. They cannot account for every aspect of individual variability nor every variable that may affect a toxicological outcome in real-world settings.
Additional Resources
AOP Training Resources
Training videos: Recorded presentations maintained by the Animal-Free Safety Assessment (AFSA) Collaboration
AOP-Wiki: This website is where AOP developers can start building new AOPs, add information to existing AOPs, or find information on established AOPs. All information is organized according to the modular AOP framework.
Original AOP Manuscript
Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. (Ankley, et al., 2010)
AOP Overview
Adverse Outcome Pathways Organizing Toxicological Information to Improve Decision Making. (Edwards, et al., 2016)
