Adverse Outcome Pathways
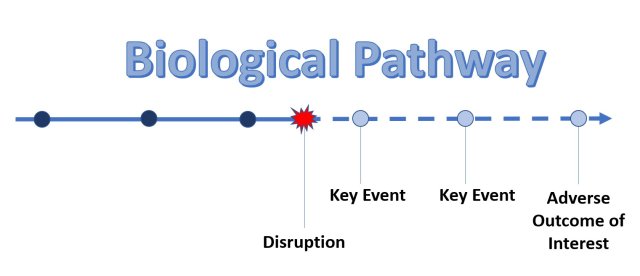
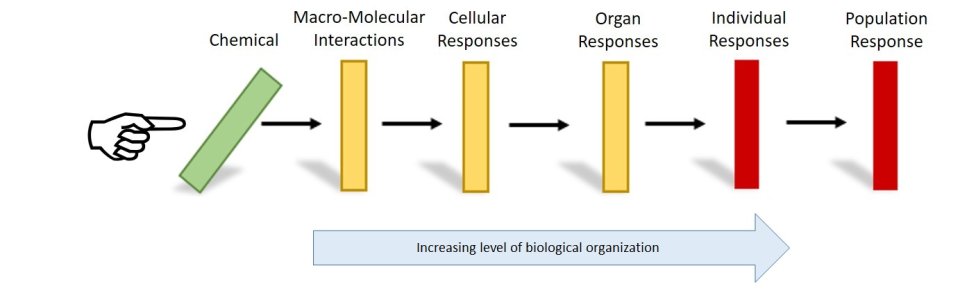
An adverse outcome pathway (AOP) describes a series of linked events at different levels of biological organization (e.g., cell, tissue, organ) that lead to an adverse health effect in an organism following exposure to a stressor (e.g., chemical). EPA researchers develop AOPs for adverse health outcomes that are relevant to risk assessment or regulatory decision making.
What is an adverse outcome?
A biological change considered relevant for risk assessment/regulatory decision making (e.g., impacts on human health/well-being or effects on survival, growth, or reproduction in wildlife).
An AOP is like a series of dominos - A chemical exposure leads to a biological change within a cell and then a “molecular initiating event” (e.g., chemical binding to DNA) triggers more dominos to fall in a cascade of sequential “key events” (e.g., abnormal cell replication) along a toxicity pathway. Together, these events can result in an adverse health outcome (e.g., cancerous cell growth) in a whole organism.
On this page:
Research Efforts
View recent AOP-related research publications
AOPs allow scientists to predict biological outcomes by extrapolating from available data. AOP networks (i.e., two or more related AOPs) can be assembled by linking individual AOPs by shared key events. The assembly of these networks provide insight into the complex interactions among biological pathways.
Because key events are measurable, researchers can use AOPs to predict the magnitude of a biological change that would need to occur before an adverse health outcome is observed. This information can help risk assessors determine how much exposure to a chemical (or chemical mixture) may lead to adverse health outcomes in a population. Learn more: What is Risk?
Building Confidence in New Approach Methods
AOPs are a critical component in the continued development and application of NAMs towards characterizing the risks for thousands of data-poor chemicals and reducing the use of animal testing.
- EPA researchers develop AOPs to build confidence in using in vitro NAMs data to predict adverse outcomes on brain development and function (neurotoxicity).
- EPA researchers use AOPs to develop in vitro methods for the more rapid identification of chemicals that cause tumors in the rat. An understanding of the AOP leading to tumor formation is building confidence in the use of these alternative methods to identify carcinogenic chemicals with less reliance on animal testing.
Priority Toxicological Endpoints
EPA researchers are working to understand the effects of chemical exposure on estrogen, androgen, and thyroid signaling (e.g., as part of the Endocrine Disruptor Screening Program), manifestations of lung toxicity, developmental neurotoxicity, and immunotoxicity endpoints.
Thyroid Disruption and Developmental Neurotoxicity
Changes in thyroid hormone levels following exposure to endocrine disrupting compounds can impact brain development. EPA researchers are conducting animal studies using AOPs to investigate key events underlying thyroid hormone dependent developmental neurotoxicity.
Toxicity of Inhaled Chemicals
EPA researchers are using quantitative AOPs to investigate the effects of inhaled reactive gases on cells of the respiratory tract leading to inflammation, abnormal cell growth, and asthma.
Children’s Environmental Health
Environmental exposures that impact health can occur before conception and during pregnancy, infancy, childhood, and adolescence. These exposures could lead to long-term effects on health, development, and risk of disease across life stages.
- EPA researchers are investigating how chemical exposures affect proteins (key events) important for human fertility, reproduction, and development. These research efforts will help to identify vulnerabilities to environmental exposures from conception to pregnancy to childhood.
Contaminants of Immediate and Emerging Concern (CIECs)
CIECs include chemical substances that may cause ecological or human health impacts and are either new or existing contaminants of increased priority.
AOPs Relevant to Per- and Polyfluoroalkyl Substances (PFAS)
EPA researchers are developing AOPs relevant to human health and ecological impacts of PFAS. These studies are evaluating applicability across species, chemical groupings, and mixtures. A wide range of adverse outcomes are being investigated, including reproductive impairment, developmental toxicity, metabolic disorders, kidney toxicity, and cardiac toxicity.
Chemical Impacts on Aquatic and Terrestrial Species
EPA researchers are using NAMs and AOPs to evaluate the ecological hazards of surface water contaminants.
Tools and Resources
- AOP Knowledge Base: Publicly accessible and searchable web-based resource of AOP information.
- AOP Wiki: A globally accessible platform for developing and disseminating AOP descriptions in accordance with international guidance and templates.
- OECD-Endorsed AOPs: The Organisation for Economic Co-operation and Development (OECD) provides guidance for development, scientific review, and OECD endorsement of AOPs.
- AOP Research Brief: Get more in-depth information on AOP research at EPA.
