Exposure Assessment Tools by Media - Aquatic Biota
Overview

Contaminated media to which people might be exposed include air, water and sediment, soil and dust, food, aquatic biota, and consumer products. Aquatic biota (fish and shellfish) can become contaminated as a result of chemical input to water bodies and subsequent uptake and accumulation in muscle, fat, and other tissues.
Inputs to water can occur from direct or indirect discharges, deposition of ambient pollutants in the air, erosion of soil and runoff flow, resuspension of chemicals from sediments, and other processes.
Human exposure to contaminants in aquatic biota can occur by direct ingestion. Various tools are available for evaluating sources and releases of contaminants to aquatic biota, fate and transport processes, and potential exposure concentrations. Exposure factors, calculation tools, and guidance for assessing exposure to contaminants in aquatic biota are also discussed in this module.
Information about ecological resources can be found on EPA’s website at:
Ecosystems Research
Information and interactive maps on fish advisories and fish tissue data are available here:
National Listing of Fish Advisories (NLFA) : Fish Tissue Data Collected by States for State Fish Advisories Search
Federal, state, and tribal links to National Listing of Fish Advisories (NLFA) contacts are provided here:
National Listing of State, Territory and Tribe Fish Advisory Contacts
U.S. Geological Survey information on aquatic resources can be found at:
Ecosystems Mission Area
Sources
Contaminants found in aquatic biota can originate from releases to air, soil, wastewater, or groundwater to surface water. Specifically, contaminants in ambient air could be deposited or washed out to aquatic systems. Erosion of contaminated soil could release chemical contaminants to surface waters.
Runoff flowing along the ground surface could transfer chemical contaminants in contaminated soil to nearby waterways. Chemical contaminants in wastewater effluents could be a source of contamination to receiving water bodies near the discharge location. In addition, chemical contaminants could leach from landfilled sewage sludge into subsoil and groundwater and migrate to surface water or sediment.
Fate and Transport
Fate and transport processes “link” the release of contaminants at a source with the resultant environmental concentrations to which receptors can be exposed. When a contaminant is released from a source, it is subject to transportMovement within a medium or between media. and transformationChange in a chemical or physical state. in the environment. Compounds can also transfer from an environmental medium to biota, a process referred to as bioconcentration or bioaccumulation.
| Migration Process | Examples Relevant to Aquatic Media |
|---|---|
| Transport |
|
| Transformation |
|
| Transfer – Environment to Biota |
|
For additional information related to the environmental fate and transport of chemical contaminants in water and sediment, refer to the Water and Sediment Module of the Media Tool Set.
Bioconcentration refers to direct transfers of the chemical from the surrounding environmental medium into the animal - it does not account for uptake by ingestion. For a fish, bioconcentration of a substance in the water includes direct uptake from water through its gills.
Bioaccumulation is the uptake of a substance through ingestion of contaminated plants or animals. In many cases, the term bioaccumulation is used as a general term to refer to the uptake of a substance from an environmental medium through both direct and indirect routes.
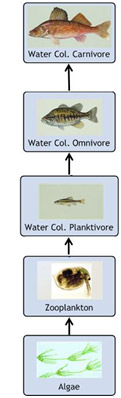
Consider a simplified fish food web. Omnivorous and carnivorous fish would accumulate more of a contaminant through their diet than planktivorous fish due to the transfer of chemicals up the food chain (i.e., through consumption of other contaminated animals). This has implications for humans who tend to eat fish that are higher in the food web.
In natural environments, the ratio of the chemical concentration in an animal to the chemical concentration in its environment (through all routes, including food chain transfers) generally is referred to as a bioaccumulation factor, or BAF. A BAF relates the concentration of a contaminant in fish tissue to the amount of chemical to which the fish is exposed through ingestion of food as well as through direct contact.
A bioconcentration factor (BCF) can be measured, but must be evaluated under controlled situations to avoid indirect uptake through the food chain since it is the ratio of chemical concentration in the animal to chemical concentration in the water only.
A biota-sediment accumulation factor, or BSAF, is analogous to a BAF. It is an empirical partitioning ratio relating concentration in sediment to the concentration in an aquatic organism, including benthic organisms and higher trophic level fish.
U.S. EPA Biota-Sediment Accumulation Factor Data is a resource for BSAFs. It contains approximately 20,000 biota-sediment accumulation factors (BSAFs) from 20 locations. Most of these are for Superfund sites, for nonionic organic chemicals and pesticides. Fresh, tidal, and marine ecosystems are included in the data.
In general, chemicals with BAFs or BCFs greater than or equal to 1,000 (equivalent to Kow of 4.2) are considered high concerns for bioaccumulation. Chemicals with values below 250 are deemed as low concerns and the rest are classified as medium concerns. BAFs/BCFs greater than 5,000 (equivalent to Kow of 5.0) indicate chemicals that are of high risk concern (U.S. EPA, 2010, 2000a).
Physicochemical/Environmental Factors
The environmental fate of a chemical contaminant in water will be dictated by its chemical and physical properties and its propensity for biotic and abiotic transformation. A summary of key physicochemical factors that are likely to affect partitioning and fate of chemical contaminants in aquatic media is provided below.
| Physicochemical Property | Definition | How Does This Property Affect Partitioning and Fate in Aquatic Media? |
|---|---|---|
| Half-life | Time required for one-half of the original mass of the chemical to be degraded, transformed, or destroyed in a particular medium. |
Values can provide an indication of persistence in sediment, surface water, or biota. For the same chemical across different media, half-lives can vary by orders of magnitude. For the purpose of defining chemicals that are persistent, EPA sets half-life criterion of > 2 months for water and sediment. Chemicals with a half-life of > 6 months are considered a “high risk concern” (U.S. EPA, 2010) EPA’s GCSOLAR is a program that computes half-lives of pollutants in the aquatic environment. |
| Vapor pressure | Indication of how likely it is that a compound will evaporate or convert from the liquid phase to the gaseous phase | The higher a chemical’s vapor pressure, the more likely that it will be found in the gas phase (and move out of water). |
| Henry’s law constant (KH) | Ratio of vapor pressure to water solubility; provides an index of partitioning for a compound between atmospheric and aqueous phases | Higher values of KH are associated with compounds that preferentially partition to air rather than to water. |
| Water solubility | Measure of the maximum amount of a chemical that will dissolve in pure water | Compounds with high solubility are likely to be mobile in water and are less likely to sorb to sediment or suspended particles in water or to bioaccumulate, and they are usually biodegradable. |
| Lipophilicity | Ability of a chemical to dissolve in fats, oils, lipids | The higher a chemical’s lipophilicity, the greater its potential to bioaccumulate in aquatic organisms (plant or animal). |
| Octanol/water partition coefficient (Kow) | Partitioning of organic chemicals between octanol (a nonaqueous, nonpolar solvent and reasonable surrogate for lipids, fat) and water | Higher Kow indicates that more of the chemical partitions into octanol (and an affinity for lipids); a lower Kow value typically correlates with a higher water solubility and suggests the compound partitions preferentially to water. The Kow is correlated with the potential for a chemical to bioaccumulate in organisms. BAFs/BCFs of 1000 and 5000 are equivalent to log Kow values of 4.2 and 5, respectively (U.S. EPA, 2010). |
| Solid/water distribution ratio (Kd) |
Ratio of the sorbed concentration (to suspended particles in water, sediment) to the concentration of the chemical dissolved in the aqueous solution | Indicates a compound’s potential to bind to sediment. Partitioning behavior will depend on the components of the solid matrix, the physical complexities of the solid matrix, and other factors. As a result, Kd is highly variable across different environments. |
| Organic carbon/water partition coefficient (Koc) | Ratio of chemical sorbed to organic carbon (component of suspended particles and sediment) to the chemical concentration dissolved in the surrounding water | For suspended or benthic sediments that have very low organic carbon, the amount of sorbed organic chemical will also be low (and dissolved concentration in water will be relatively high) and vice versa. |
Characteristics of the aquatic environment-including surface water flow rate, temperature, and pH, as well as meteorological factors such as sunlight and precipitation-can also impact fate and transport. For example, water solubility is a pH- and temperature-dependent parameter. In general, as temperature increases, the solubility of given solid increases. In contrast, the solubility of a gas generally decreases with increasing temperature.
Acidic (low pH) conditions in surface water tend to increase the solubility of metal salts that might otherwise precipitate out of solution. So, acidic conditions can lead to higher dissolved concentrations of some metals, sometimes resulting in more toxic conditions for organisms living in the water.
Organic vs. Inorganic and PBTs
Organic compounds that enter water can be transformed in two ways-by abiotic or biotic processes.
- During abiotic processes, compounds chemically react and degrade in the environment through reactions initiated or assisted by exposure to light, water, or oxygen.
- During biotic processes (biodegradation), breakdown of the chemical occurs by a biotic organism, such as bacteria. The reaction or degradation products can themselves be contaminants of concern.
(See the Other Organics Module of the Chemical Classes Tool Set for more information and resources for assessing exposure to organic compounds.)
Inorganic compounds also undergo transformation reactions, including some that are similar to the chemical and biological reactions that involve organic compounds. For example, reactions with light, water, or oxygen might occur. However, inorganic compounds cannot be broken down beyond the metal or other species that is the basis of the compound. That is, they cannot be “completely” degraded.
(See the Inorganics and Fibers Module of the Chemical Classes Tool Set for more information and resources for assessing exposure to inorganic compounds.)
Changes in speciation and complexation reactions are important for inorganics. These can result from reduction/oxidation reactions (redox reactions) that change the valence of the inorganic species and can affect the solubility, mobility, or other characteristics of the substance.
Precipitation and dissolution of inorganic compounds in water are also important processes that directly affect the subsequent fate and transport of metal salts and other compounds.
Persistent, bioaccumulative, and toxic (PBT) contaminants are chemicals that are persistent in the environment, bioaccumulate in food chains, and are toxic, posing risks to aquatic systems and human consumers of aquatic biota. For the purpose of defining chemicals that are persistent, EPA sets a biodegradation half-life criterion of >2 months for water and sediment. Chemicals with a half-life of >6 months in water and sediment are considered a "high risk concern." Contaminants with a Kow value that is ≥4.2 are considered to have high bioaccumulation potential.
Toxic chemicals are those that are associated with a range of adverse human health effects, including effects on the nervous system, reproductive and developmental problems, cancer, and genetic impacts. They also have the potential to pose a risk via food chain toxicity.
EPA priority PBTs include polychlorinated biphenyls (PCBs), dioxins, various pesticides (e.g., aldrin, dieldrin, chlordane, DDT, hexachlorobenzene, mirex, toxaphene), mercury, and alkyl-lead.
PCBs, dioxins, and many pesticides are semivolatile organic compounds meaning they have slow volatilization rates from the solids (e.g., sediments) or liquids (e.g., water) that contain them. They have low solubility in water and exist mostly sorbed to particles (i.e., sediment, suspended materials in water).
EPA has identified PCBs as an important chemical risk concern from fish consumption because they are so resistant to breakdown in the environment. Also, exposure can result in health effects in humans. PCBs build up in fish to levels hundreds of thousands of times higher than the levels in water.
In general, very low levels of dioxins are found in water. In aquatic systems, dioxins undergo sedimentation and burial in aquatic sediments where degradation then occurs at a very slow rate. Dioxins are very persistent and highly lipophilic, so they have great potential to bioaccumulate in aquatic organisms. They can also biomagnify up through the food chain.
The Other Organics Module of the Chemical Classes Tool Set provides information and resources on assessing exposure to PCBs and dioxins.
Some pesticides are very resistant to breakdown and biomagnify up through the food chain. For example, it can take more than 15 years for DDT to break down in the environment. DDT breakdown products (i.e., DDD and DDE) are also PBT pollutants.
Many of the PBT pesticides such as DDT, dieldrin, chlordane, hexachlorobenzene, and mirex are banned from use in the United States since the 1970s or 1980s. However, due their persistence, they are still lingering in the environment. See the Pesticides Module of the Chemical Classes Tool Set for more information and resources on assessing exposure to pesticides.
Burning coal leads to emissions of elemental mercury and divalent mercury. Divalent mercury can deposit to surface water, where it can be transformed to methyl mercury (MeHg) by anaerobic microbes. This chemical transformation is of particular concern because MeHg readily bioaccumulates in fish (unlike divalent mercury), and MeHg is a potent neurotoxin in humans.
MeHg builds up more in some types of fish and shellfish than others, depending on what the fish eat, which is why the levels in species vary. MeHg accumulates in the muscle tissue of the fillet and cannot be removed by trimming fat from the fish or cooking it, adding to the potential for exposure. EPA provides information related to the fate and transport of mercury in aquatic environments in Volume III of its 1997 Mercury Study Report to Congress.
Inorganic lead may bioconcentrate in some aquatic biota, particularly benthic organisms such as bottom feeding fish and shellfish like mussels. Biomagnification of inorganic lead is not believed to be significant in aquatic organisms. However, alkyl-lead compounds might significantly accumulate in both fish and shellfish. Alkyl-lead accumulates in "soft tissues" particularly the liver, kidneys, muscles, and brain.
The Inorganics and Fibers Module of the Chemical Classes Tool Set provides information and resources on assessing exposure to mercury and lead.Models
Environmental models can help to inform the fate and transport and the environmental concentration components of exposure assessment. Monitoring data can be used with environmental fate and transport models to better characterize exposure concentrations for aquatic media. When measured concentrations are not available, models can be used to estimate media concentrations and potential exposure concentrations in lieu of environmental data.
A bioaccumulation model can be used to predict fish tissue concentrations as a simple linear product of food or media concentrations and a bioaccumulation factor. A bioenergetics model is a type of bioaccumulation model that accounts for the exchange of chemical mass between multiple levels of a food web (i.e., biomagnification). Models that may be useful in estimating uptake and bioaccumulation of chemicals in aquatic biota are described below.
Concentrations
Concentrations of contaminants in aquatic biota may be determined based on measurements or modeling. Characterizing contaminant concentrations for an exposure scenario is typically accomplished using some combination of the following approaches:
- Sampling fish or other aquatic biota and measuring contaminant concentrations in tissues
- Modeling the concentrations based on source strength, media transport, and chemical transformation processes
- Using existing, available measured concentration data collected for related analysis or compiled in databases
EPA provides information on measuring or modeling aquatic biota concentrations and on available monitoring data. Information on sampling methods is available to support the measurement of contaminants in aquatic biota. In the absence of monitoring data, a variety of models can be used to estimate contaminant concentrations in aquatic biota based on contaminant transport from other media such as surface water and sediment.
Fish consumption advisories are another important factor affecting human exposure to contaminants in fish. They provide warnings to human consumers to avoid consumption of certain kinds or certain amounts of fish contaminated by PBTs and other toxic chemicals.
If consumers heed these warnings, then their exposure to contaminant concentrations in these fish will be reduced. PBTs such as mercury, PCBs, dioxins, and pesticides such as DDT are responsible for most national fish consumption advisories.
Fish consumption advisories are compiled in EPA’s Fish Consumption Advisories website, which describes state, tribal, and federally- issued fish and wildlife consumption advisories in the United States and U.S. territories as well as the Canadian provinces and territories.
In addition, EPA's Fish Consumption Advisory website provides a compilation of information on locally issued fish advisories and safe eating guidelines. It includes access to the National Listing of Fish Advisories (NLFA), an interactive mapping and search tool that can be used to view information about fish advisories based on the geographic location of a water body, the species of the fish, the chemical contaminants identified in the advisory, and the portion of the consumer population for whom the advisory was issued.
NLFA is searchable by state or U.S. territory and water body. Consumption restrictions are indicated as red (fish advisory), purple (safe eating guidelines with some restrictions), or green (safe eating guidelines with no restrictions). Detailed advisory reports provide species of fish, the affected population of consumers, and which contaminant(s) triggered the advisory.
State environmental programs and public health departments also issue fish consumption advisories for their state waterbodies. Links to state fish advisory websites and state contact information are available from the NLFA webpage. NLFA information is updated about every two years, so the complete and most up-to-date information should be obtained from the state websites.
Measuring Concentrations
A determination of the concentration of contaminants in aquatic media may be based on measurements or modeling. Information on sampling techniques or protocols and analytical methods can be used to support the measurement of contaminants in aquatic biota.
Modeling Concentrations
In the absence of monitoring data, models may be used to estimate the concentrations of contaminants in water and aquatic biota, such as the following.
Available Data
There are a number of information sources that provide monitoring data on contaminant concentrations in surface water, sediment, and aquatic biota, including the following.
Exposure Scenarios
Exposure to contaminants in fish can be estimated by first defining the exposure scenario of interest. Exposure scenarios typically include information on the sources and pathways of exposure, contaminants of concern, and receptor populations. They might also describe a receptor population’s activities that may affect exposure and the timeframe over which exposure occurs.
Ingestion scenarios are intended to cover routes by which bioaccumulative chemicals might end up in the food chain. Fish ingestion is a commonly evaluated scenario. Persistent bioaccumulative (lipophilic) chemicals can accumulate in fish following deposition or release to the water body and watershed and transfer to fish via diet and direct transfer from the water. For a fish ingestion scenario, concentrations of the contaminants in fish (modeled or measured) are needed to estimate exposure dose.
A member of the general population could be consuming contaminated fish although the percentage of contaminated fish consumed might be diluted if fish from a variety of sources are consumed. Other important receptor populations for fish ingestion include subsistence (e.g., Native American) fishers and recreational anglers who may be more vulnerable to exposure via intake of contaminated fish from water bodies at specific locations.
Subsistence fishers might have increased vulnerability given that the fish they catch could be a primary source of food for themselves or for their families. In addition, some ethnic groups are more likely to eat fish skin, cooking juices, and raw fish than other anglers. This may make them more vulnerable to portions of the fish that can contain higher levels of contamination.
After identifying exposure concentrations and characterizing the exposed population, it is important to define all appropriate exposure factor inputs to use to estimate potential exposures and risks. These inputs (intake rates and other relevant patterns of behavior) can be obtained from the Exposure Factors Handbook: 2011 Edition (see Exposure Factors tab in the Indirect Estimation Module of Approaches).
The table below provides some examples of scenarios involving contaminants in fish and shellfish. The list of examples is not meant to be exhaustive. There are numerous other fish and shellfish ingestion scenarios that may be constructed based on the specific needs of the assessment. There are also numerous variations of the examples provided in the table.
Additional information on exposure scenarios involving contaminated fish and shellfish may be found in the Indirect Estimation Module of the Approaches Tool Set in EPA ExpoBox.
| Medium/Route | Receptor Population | Activity Pattern | Data Type | Exposure Period |
|---|---|---|---|---|
| Total finfish and shellfish ingestion | General population, per capita |
Fish obtained from all sources | Body weight-normalized daily intake (g/kg-day) for whole population and by age group and race [Table 10-11] |
Subchronic or chronic |
| Total finfish and shellfish ingestion | General population, consumer-only | Fish obtained from all sources | Body weight-normalized daily intake (g/kg-day) for whole population and by age group and race [Tables 10-11, 10-12] |
Subchronic or chronic |
| Fish (general) ingestion | General population, per capita |
Fish obtained from all sources | Body weight-normalized daily intake (g/kg-day) for all survey respondents and by geographic location, age group, sex, race, and various sociodemographic variables [Tables 10-11, 10-37] |
Subchronic or chronic |
| Fish (general) ingestion | General population, consumer-only | Fish obtained from all sources | Body weight-normalized daily intake (g/kg-day) for all survey respondents and by geographic location, age group, sex, race, and various sociodemographic variables [Tables 10-11, 10-38] |
Subchronic or chronic |
| Finfish ingestion | Recreational marine anglers | Fish that are self-caught | Daily intake (g/day) for all anglers by geographic region [Tables 10-11, 10-50] |
Chronic |
| Finfish and shellfish ingestion | Recreational marine anglers and their families | Fish that are self-caught | Number of fish meals and portion sizes for anglers and their families in Lavaca, Texas [Tables 10-11, 10-62] |
Subchronic or chronic |
| Fish (general) ingestion | Recreational freshwater anglers | Fish that are self-caught | Number of fish meals, daily intake (g/day), and body weight-normalized daily intake (g/kg-day) for anglers in Michigan [Tables 10-11, 10-71] |
Chronic |
| Selected fish species ingestion | Recreational freshwater anglers | Fish that are self-caught | Total amount of fish consumed (g) by species for anglers in Maine in 1990 [Tables 10-11, 10-74] |
Chronic |
| Fish (general) ingestion | Native American populations, consuming and nonconsuming adults | Fish that are self-caught | Daily intake (g/day) for adult anglers from tribes of the Columbia River Basin [Tables 10-11, 10-88] |
Chronic |
| Fish (general) ingestion | Native American populations, consuming and nonconsuming children ≤ 5 years | Fish that are self-caught | Daily intake (g/day) for the young children of anglers from tribes of the Columbia River Basin [Tables 10-11, 10-90] |
Subchronic |
Several resources are available that illustrate fish ingestion exposure scenarios.
Exposure Factors
To estimate human exposure to contaminants in aquatic biota, exposure factor information is needed. Exposure factors are human behaviors and characteristics that help determine an individual's exposure to an agent.
Data on fish and shellfish ingestion rates are available in Chapter 10 of EPA’s Exposure Factors Handbook: 2011 Edition. Intake rates are provided in units of g/day or g/kg-day (normalized to body weight). Many of the study-specific intake rate summaries reflect the distribution in the target population, so mean, median, and other percentiles intake rates are often presented.
At the beginning of Chapter 10, recommended values for fish intake are summarized for the general population and recreational marine anglers. Recommended values are not provided for recreational freshwater anglers or Native American fishers because intake data are limited to specific geographic areas for these receptor groups. However, data from relevant studies are presented.
Intake rate recommendations for the general population are reported as uncooked fish weights because contaminant concentrations in fish are typically measured in uncooked fish. Chapter 10 also provides “as-prepared” (i.e., as-consumed) consumption rates for the general population in cases where concentration data are adjusted to account for changes after cooking.
Intake rates are provided as per capita or consumer-only data. Consumer-only rates are intake rates pertaining only to those individuals who reported eating the foods during the surveyed period. Per capita rates are defined as rates pertaining to the whole study population, and include both individuals who ingested the food during the survey period and individuals who did not.
Per capita intake rates may be used in exposure assessments of the general population for which average dose estimates are of interest. Consumer-only intake rates are more pertinent for assessing specific more vulnerable populations (e.g., subsistence fishers).
The abundance of data in Chapter 10 reflects the variation in consumption rates that are available from relevant studies. Intake rates in Chapter 10 are organized based on the following distinctions.
- Species category. The focus of Chapter 10 is on fish—specifically, finfish and shellfish. However, the studies considered and data presented are not limited to these groups. Intake values are also provided for seafood. Data for other defined groups (e.g., anadromous, pelagic, bottom fish) and a wide range of individual species are provided.
- Acquisition method. In some cases, intake rates are identified based on whether the fish was purchased or self-caught.
- Characteristics of the survey population. Because intake rates can vary across different receptor groups, rates are presented for the general population, recreational anglers, and Native American fishers and have also been described for characteristics of the individual, including:
- Gender
- Age (infant, child, adult, and older adult age groups) or life stage (e.g., pregnant or lactating woman, woman of childbearing age)
- Health status (e.g., asthma, angina, cigarette smoking, alcohol consumption). Individuals with pre-existing conditions could be more susceptible to the contaminants they consume in fish.
- Type of water body. Some intake rates are provided for species obtained from marine waters, freshwater/estuarine waters, or the combined habitats.
- Sociodemographic variables. Some intake rates are summarized by race/ethnicity, education level, income level, and similar variables. Specific racial, ethnic, and socioeconomic groups might be disproportionately exposed to certain chemicals due to differences in diets and cultural activities.
- Spatial/temporal factors. Intake rates are often defined for specific geographic regions (particularly for recreational anglers and Native Americans), and also consider urban v. rural locations, day of the week, and season.
Selection of intake rates considering the appropriate category (or categories) above will be dictated by the exposure scenario being evaluated. In addition to intake rates, studies that describe serving size, fish parameter values (fish weight, fat content, moisture content), and the percent use of particular fish preparation/cooking methods are provided.
Other exposure factors that might be needed for assessing ingestion exposures include:
- Body weight (Chapter 8)
- Life expectancy values, specifically when evaluating cancer risk (Chapter 18)
Exposure factor data may be accessed from the Exposure Factors Tab of the Indirect Estimation Module.
Calculation Tools
A variety of tools are available for quantifying exposures (dose) and risks associated with contaminants in aquatic biota. These calculation tools have typically been developed for specific situations or program offices but may be tailored to meet the needs of the user. The resources listed below include tools that might be used to calculate dose/risk to aquatic biota as well as documents that describe how to quantify dose/risk or provide reference values/benchmarks for assessing risks.
Calculation Tools – Human Receptors
Calculation Tools – Ecological Receptors
Guidance
A number of guidance documents are available to support various components of EPA programs involving aquatic biota.
References
- U.S. EPA. Mercury Study Report to Congress. Washington, DC.
- U.S. EPA. (2017). Water Quality Standards Handbook: Chapter 3: Water Quality Criteria. EPA-823-B-17-001. EPA Office of Water, Office of Science and Technology, Washington, DC.
- U.S. EPA. (2000a). Bioaccumulation Testing and Interpretation for the Purpose of Sediment Quality Assessment, Status and Needs. (EPA/823/R-00/001). Washington, DC.
- U.S. EPA. (2000b). National Guidance: Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories - Volume 2: Risk Assessment and Fish Consumption Limits: Third Edition. (EPA/823/B-00/008). Washington, DC.
- U.S. EPA. (2010). TSCA New Chemicals Program (NCP) Chemical Categories. Washington, DC.
